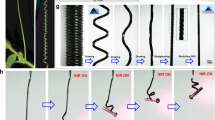
Abstract
A key goal of nanotechnology is the development of artificial machines capable of converting molecular movement into macroscopic work. Although conversion of light into shape changes has been reported and compared to artificial muscles, real applications require work against an external load. Here, we describe the design, synthesis and operation of spring-like materials capable of converting light energy into mechanical work at the macroscopic scale. These versatile materials consist of molecular switches embedded in liquid-crystalline polymer springs. In these springs, molecular movement is converted and amplified into controlled and reversible twisting motions. The springs display complex motion, which includes winding, unwinding and helix inversion, as dictated by their initial shape. Importantly, they can produce work by moving a macroscopic object and mimicking mechanical movements, such as those used by plant tendrils to help the plant access sunlight. These functional materials have potential applications in micromechanical systems, soft robotics and artificial muscles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Feynman, R. P. in Miniaturization (ed. Gilbert, H. D.) 282–296 (Reinhold, 1961).
Drexler, K. E. Nanosystems: Molecular Machinery, Manufacturing and Computation (Wiley, 1992).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Fletcher, S. P., Dumur, F., Pollard, M. M. & Feringa, B. L. A reversible, unidirectional molecular rotary motor driven by chemical energy. Science 310, 80–82 (2005).
Balzani, V. et al. Autonomous artificial nanomotor powered by sunlight. Proc. Natl Acad. Sci. USA 103, 1178–1183 (2006).
von Delius, M., Geertsema, E. M. & Leigh, D. A. A synthetic small molecule that can walk down a track. Nature Chem. 2, 96–101 (2010).
Muraoka, T., Kinbara, K. & Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 440, 512–515 (2006).
Kudernac, T. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 479, 208–211 (2011).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
Berná, J. et al. Macroscopic transport by synthetic molecular machines. Nature Mater. 4, 704–710 (2005).
Eelkema, R. et al. Nanomotor rotates microscale objects. Nature 440, 163 (2006).
Yamada, M. et al. Photomobile polymer materials: towards light-driven plastic motors. Angew. Chem. Int. Ed. 47, 4986–4988 (2008).
Juluri, B. K. et al. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano 3, 291–300 (2009).
Morimoto, M. & Irie, M. A diarylethene cocrystal that converts light into mechanical work. J. Am. Chem. Soc. 132, 14172–14178 (2010).
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nature Nanotechnol. 1, 25–35 (2006).
Ariga, K., Mori, T. & Hill, J. P. Mechanical control of nanomaterials and nanosystems. Adv. Mater. 24, 158–176 (2012).
Coskun, A. et al. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Spinks, G. M. Deforming materials with light: photoresponsive materials muscle in on the action. Angew. Chem. Int. Ed. 51, 2285–2287 (2012).
Schliwa, M. Molecular Motors (Wiley, 2003).
Mahadevan, L. & Matsudaira, P. Motility powered by supramolecular springs and ratchets. Science 288, 95–99 (2000).
Armon, S., Efrati, E., Kupferman, R. & Sharon, E. Geometry and mechanics in the opening of chiral seed pods. Science 333, 1726–1730 (2011).
Gerbode, S. J., Puzey, J. R., McCormick, A. G. & Mahadevan, L. How the cucumber tendril coils and overwinds. Science 337, 1087–1091 (2012).
Zhu, L., Al-Kaysi, R. O. & Bardeen, C. J. Reversible photoinduced twisting of molecular crystal microribbons. J. Am. Chem. Soc. 133, 12569–12575 (2011).
Kitagawa, D., Nishi, H. & Kobatake, S. Photoinduced twisting of a photochromic diarylethene crystal. Angew. Chem. Int. Ed. 52, 9320–9322 (2013).
Lee, K. M. et al. Photodriven, flexural–torsional oscillation of glassy azobenzene liquid crystal polymer networks. Adv. Funct. Mater. 21, 2913–2918 (2011).
Warner, M. & Terentjev, E. M. Liquid Crystal Elastomers (Oxford Univ. Press, 2003).
Natansohn, A. & Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 102, 4139–4175 (2002).
Yu, Y., Nakano, M. & Ikeda, T. Directed bending of a polymer film by light. Nature 425, 145 (2003).
Van Oosten, C. L., Bastiaansen, C. W. M. & Broer, D. J. Printed artificial cilia from liquid-crystal network actuators modularly driven by light. Nature Mater. 8, 677–682 (2009).
Van Oosten, C. L., Harris, K. D., Bastiaansen, C. W. M. & Broer, D. J. Glassy photomechanical liquid-crystal network actuators for microscale devices. Eur. Phys. J. E 23, 329–336 (2007).
Sawa, Y. et al. Shape selection of twist-nematic-elastomer ribbons. Proc. Natl Acad. Sci. USA 108, 6364–6368 (2011).
Sawa, Y. et al. Shape and chirality transitions in off-axis twist nematic elastomer ribbons, Phys. Rev. E 88, 022502 (2013).
Teresi, L. & Varano, V. Modeling helicoid to spiral-ribbon transitions of twist-nematic elastomers. Soft Mater 9, 3081–3088 (2013).
Forterre, Y. & Dumais, J. Generating helices in nature. Science 333, 1715–1716 (2011).
van Oosten, C. L. et al. Bending dynamics and directionality reversal in liquid crystal network photoactuators. Macromolecules 41, 8592–8596 (2008).
Harris, K. D. et al. Large amplitude light-induced motion in high elastic modulus polymer actuators. J. Mater. Chem. 15, 5043–5048 (2005).
Hosono, N. et al. Large-area three-dimensional molecular ordering of a polymer brush by one-step processing. Science 330, 808–811 (2010).
Mossety-Leszczak, B., Wlodarska, M., Galina, H. & Bak, G. W. Comparing liquid crystalline properties of two epoxy compounds based on the same azoxy group. Mol. Cryst. Liq. Cryst. 490, 52–66 (2008).
Li, C. et al. Synthesis of a photoresponsive liquid-crystalline polymer containing azobenzene. Macromol. Rapid Commun. 30, 1928–1935 (2009).
Acknowledgements
This work was supported financially by the European Research Council (Starting Grant 307784 to N.K.), the Netherlands Organisation for Scientific Research (a Vidi Grant to N.K.) and The Royal Society UK (an International Exchanges Grant to S.P.F. & N.K.).
Author information
Authors and Affiliations
Contributions
N.K. and S.P.F. conceived the research. N.K., T.K. and J.L.M.C. guided the research. S.I. and B.M. synthesized 1. S.I., S.J.A. and B.M. carried out the experiments. All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1819 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 10358 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 23260 kb)
Supplementary Movie 3
Supplementary Movie 3 (MOV 7070 kb)
Supplementary Movie 4
Supplementary Movie 4 (WMV 28990 kb)
Rights and permissions
About this article
Cite this article
Iamsaard, S., Aßhoff, S., Matt, B. et al. Conversion of light into macroscopic helical motion. Nature Chem 6, 229–235 (2014). https://doi.org/10.1038/nchem.1859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1859
This article is cited by
-
Functionally antagonistic polyelectrolyte for electro-ionic soft actuator
Nature Communications (2024)
-
Photoswitchable gating of non-equilibrium enzymatic feedback in chemically communicating polymersome nanoreactors
Nature Chemistry (2023)
-
Polarization-driven reversible actuation in a photo-responsive polymer composite
Nature Communications (2023)
-
Bi-enzymatic chemo-mechanical feedback loop for continuous self-sustained actuation of conducting polymers
Nature Communications (2023)
-
Light moves artificial cilia to a complex beat
Nature (2022)