Abstract
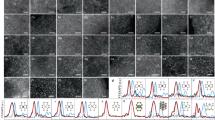
Identifying and understanding the active sites responsible for reaction turnover is critical to developing improved catalysts. For the hydrogen-evolution reaction (HER), MoS2 has been identified as an active non-noble-metal-based catalyst. However, only edge sites turnover the reaction because the basal planes are catalytically inert. In an effort to develop a scalable HER catalyst with an increased number of active sites, herein we report a Mo–S catalyst (supported thiomolybdate [Mo3S13]2− nanoclusters) in which most sulfur atoms in the structure exhibit a structural motif similar to that observed at MoS2 edges. Supported sub-monolayers of [Mo3S13]2− nanoclusters exhibited excellent HER activity and stability in acid. Imaging at the atomic scale with scanning tunnelling microscopy allowed for direct characterization of these supported catalysts. The [Mo3S13]2− nanoclusters reported herein demonstrated excellent turnover frequencies, higher than those observed for other non-precious metal catalysts synthesized by a scalable route.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
US Energy Information Administration. The Impact of Increased Use of Hydrogen on Petroleum Consumption and Carbon Dioxide Emissions (US Energy Information Administration, 2008).
Turner, J. A. Sustainable hydrogen production. Science 305, 972–974 (2004).
Nowotny, J., Sorrell, C. C., Sheppard, L. R. & Bak, T. Solar-hydrogen: environmentally safe fuel for the future. Int. J. Hydrogen Energy 30, 521–544 (2005).
Chen, Z. et al. Accelerating materials development for photoelectrochemical hydrogen production: standards for methods, definitions, and reporting protocols. J. Mater. Res. 25, 3–16 (2010).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Choquette, Y., Brossard, L., Lasia, A. & Ménard, H. Investigation of hydrogen evolution on Raney–Nickel composite-coated electrodes. Electrochim. Acta 35, 1251–1256 (1990).
Lačnjevac, U. Č., Jović, B. M., Jović, V. D. & Krstajić, N. V. Determination of kinetic parameters for the hydrogen evolution reaction on the electrodeposited Ni–MoO2 composite coating in alkaline solution. J. Electroanal. Chem. 677–680, 31–40 (2012).
McKone, J. R., Sadtler, B., Werlang, C. A., Lewis, N. S. & Gray, H. B. Ni–Mo nanopowder for efficient electrochemical hydrogen evolution. ACS Catal. 3, 166–169 (2013).
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Karunadasa, H. I. et al. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335, 698–702 (2012).
Li, Y. et al. MoS2 Nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Xiang, Q. J., Yu, J. G. & Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 134, 6575–6578 (2012).
Chen, Z. et al. Core–shell MoO3–MoS2 nanowires for hydrogen evolution: a functional design for electrocatalytic materials. Nano Lett. 11, 4168–4175 (2011).
Kibsgaard, J., Chen, Z., Reinecke, B. N. & Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Mater. 11, 963–969 (2012).
Merki, D., Fierro, S., Vrubel, H. & Hu, X. L. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 (2011).
Benck, J. D., Chen, Z. B., Kuritzky, L. Y., Forman, A. J. & Jaramillo, T. F. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: insights into the origin of their catalytic activity. ACS Catal. 2, 1916–1923 (2012).
Le Goff, A. et al. From hydrogenases to noble metal-free catalytic nanomaterials for H2 production and uptake. Science 326, 1384–1387 (2009).
Helm, M. L., Stewart, M. P., Bullock, R. M., DuBois, M. R. & DuBois, D. L. A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s−1 for H2 production. Science 333, 863–866 (2011).
Andreiadis, E. S. et al. Molecular engineering of a cobalt-based electrocatalytic nanomaterial for H2 evolution under fully aqueous conditions. Nature Chem. 5, 48–53 (2013).
Mondal, B. et al. Cobalt corrole catalyst for efficient hydrogen evolution reaction from H2O under ambient conditions: reactivity, spectroscopy, and density functional theory calculations. Inorg. Chem. 52, 3381–3387 (2013).
Duval, S. et al. Capture of the [Mo3S4]4+ cluster within a {Mo18} macrocycle yielding a supramolecular assembly stabilized by a dynamic H-bond network. J. Am. Chem. Soc. 132, 2069–2077 (2010).
Hijazi, A. et al. Tuning the electrocatalytic hydrogen evolution reaction promoted by [Mo2O2S2]-based molybdenum cycles in aqueous medium. Dalton Trans. 42, 4848–4858 (2013).
McKone, J. R., Gray, H. B. & Lewis, N. S. Will solar-driven water-splitting devices see the light of day? Chem. Mater. http://dx.doi.org/10.1021/cm4021518 (2013).
Müller, A., Krickemeyer, E., Hadjikyriacou, A. & Coucouvanis, D. in Inorganic Syntheses Vol. 27 (ed. Ginsberg, A.P.) 47–51 (John Wiley, 2007).
Müller, A., Wittneben, V., Krickemeyer, E., Bogge, H. & Lemke, M. Studies on the triangular cluster [Mo3S13]2−: electronic structure (Xa calculations, XPS), crystal structure of (Ph4,As)2[Mo3S13]·2CH3CN and a refinement of the crystal structure of (NH4)2[Mo3S13]·H2O. Z. Anorg. Allg. Chem. 605, 175–188 (1991).
Leist, A. et al. Semiporous MoS2 obtained by the decomposition of thiomolybdate precursors. J. Mater. Chem. 8, 241–244 (1998).
Boudart, M. Turnover rates in heterogeneous catalysis. Chem. Rev. 95, 661–666 (1995).
Zambelli, T., Wintterlin, J., Trost, J. & Ertl, G. Identification of the ‘active sites’ of a surface-catalyzed reaction. Science 273, 1688–1690 (1996).
Vang, R. T., Lauritsen, J. V., Laegsgaard, E. & Besenbacher, F. Scanning tunneling microscopy as a tool to study catalytically relevant model systems. Chem. Soc. Rev. 37, 2191–2203 (2008).
Helveg, S. et al. Atomic-scale structure of single-layer MoS2 nanoclusters. Phys. Rev. Lett. 84, 951–954 (2000).
Lauritsen, J. V. et al. Size-dependent structure of MoS2 nanocrystals. Nature Nanotech. 2, 53–58 (2007).
Sun, D. et al. An MoSx structure with high affinity for adsorbate interaction. Angew. Chem. Int. Ed. 51, 10284–10288 (2012).
Muijsers, J. C., Weber, T., Vanhardeveld, R. M., Zandbergen, H. W. & Niemantsverdriet, J. W. Sulfidation study of molybdenum oxide using MoO3/SiO2/Si(100) model catalysts and Mo3IV–sulfur cluster compounds. J. Catal. 157, 698–705 (1995).
Weber, T., Muijsers, J. C. & Niemantsverdriet, J. W. Structure of amorphous MoS3 . J. Phys. Chem. 99, 9194–9200 (1995).
Lefebvre, M. in Modern Aspects of Electrochemistry Vol. 32 (eds Conway, B. E., Bockris J. O. M. & White, R.) 249–300 (Springer, 2002).
Kodintsev, I. M. & Trasatti, S. Electrocatalysis of H2 evolution on RuO2+IrO2 mixed oxide electrodes. Electrochim. Acta 39, 1803–1808 (1994).
Jaramillo, T. F. et al. Hydrogen evolution on supported incomplete cubane-type [Mo3S4]4+ electrocatalysts. J. Phys. Chem. C 112, 17492–17498 (2008).
Chen, W-F. et al. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012).
Bonde, J., Moses, P. G., Jaramillo, T. F., Norskov, J. K. & Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 140, 219–231 (2008).
Merki, D., Vrubel, H., Rovelli, L., Fierro, S. & Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 3, 2515–2525 (2012).
Tran, P. D. et al. Novel cobalt/nickel–tungsten–sulfide catalysts for electrocatalytic hydrogen generation from water. Energy Environ. Sci. 6, 2452–2459 (2013).
Tran, P. D. et al. Copper molybdenum sulfide: a new efficient electrocatalyst for hydrogen production from water. Energy Environ. Sci. 5, 8912–8916 (2012).
Acknowledgements
J.K. gratefully acknowledges the Carlsberg Foundation for a postdoctoral fellowship. J.K. and T.F.J. acknowledge support from the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-SC0008685. We thank Z. Chen for helpful discussion and A. V. Malkovskiy for assistance with Raman spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
J.K. conceived the studies and performed the experimental work. J.K., T.F.J. and F.B. conducted data analysis and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6747 kb)
Rights and permissions
About this article
Cite this article
Kibsgaard, J., Jaramillo, T. & Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nature Chem 6, 248–253 (2014). https://doi.org/10.1038/nchem.1853
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1853
This article is cited by
-
Integrated interfacial design of covalent organic framework photocatalysts to promote hydrogen evolution from water
Nature Communications (2023)
-
Moiré superlattice engineering of two-dimensional materials for electrocatalytic hydrogen evolution reaction
Nano Research (2023)
-
Amorphous NiMo3S13/nickel foam integrated anode for lithium-ion batteries
Tungsten (2023)
-
Syntheses, Characterizations and Properties of [Mo2O2S2]-Based Oxothiomolybdenum Rings Incorporating Carboxylate Ligands
Journal of Cluster Science (2023)
-
Observation of polarity-switchable photoconductivity in III-nitride/MoSx core-shell nanowires
Light: Science & Applications (2022)