Abstract
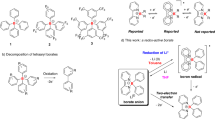
Many metal-containing compounds, and some metal-free compounds, will bind carbon monoxide. However, only a handful of metal-containing compounds have been shown to induce the coupling of two or more CO molecules, potentially a method for the use of CO as a one-carbon-atom building block for the synthesis of organic molecules. In this work, CO was added to a boron–boron triple bond at room temperature and atmospheric pressure, resulting in a compound into which four equivalents of CO are incorporated: a flat, bicyclic, bis(boralactone). By the controlled addition of one CO to the diboryne compound, an intermediate in the CO coupling reaction was isolated and structurally characterized. Electrochemical measurements confirm the strongly reducing nature of the diboryne compound.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gmelin, L. Über einige merkwürdige, bei der Darstellung des Kaliums nach der Brunnerschen Methode, erhaltene Substanzen. Ann. Phys. Chem. 4, 31–62 (1825).
Liebig, J. Über das Verhalten des Kohlenoxyds zu Kalium. Ann. Pharm. 11, 182–189 (1834).
Evans, W. J., Grate, J. W., Hughes, L. A., Zhang, H. & Atwood, J. L. Reductive homologation of CO to a ketenecarboxylate by a low-valent organolanthanide complex—synthesis and X-ray crystal-structure of [(C5Me5)4Sm2(O2CCCO)(THF)]2 . J. Am. Chem. Soc. 107, 3728–3730 (1985).
Evans, W. J., Lee, D. S., Ziller, J. W. & Kaltsoyannis, N. Trivalent [(C5Me5)2(THF)Ln]2(μ-η2:η2-N2) complexes as reducing agents including the reductive homologation of CO to a ketene carboxylate, (μ-η4-O2C–C=C=O)2–. J. Am. Chem. Soc. 128, 14176–14184 (2006).
Bianconi, P. A. et al. Reductive coupling of carbon monoxide ligands to form coordinated bis(trimethylsiloxy)ethyne in seven-coordinate niobium(I) and tantalum(I) [M(CO)2(dmpe)2Cl] complexes. Organometallics 6, 1968–1977 (1987).
Summerscales, O. T., Cloke, F. G. N., Hitchcock, P. B., Green, J. C. & Hazari, N. Reductive cyclotrimerization of carbon monoxide to the deltate dianion by an organometallic uranium complex. Science 311, 829–831 (2006).
Summerscales, O. T., Cloke, F. G. N., Hitchcock, P. B., Green, J. C. & Hazari, N. Reductive cyclotetramerization of CO to squarate by a U(III) complex: the X-ray crystal structure of [(U(η-C8H6{(SiPr3)-Pr-i-1,4}2)(η-C5Me4H)]2(μ-η2:η2-C4O4). J. Am. Chem. Soc. 128, 9602–9603 (2006).
Frey, A. S. et al. Mechanistic studies on the reductive cyclooligomerisation of CO by U(III) mixed sandwich complexes; the molecular structure of [U(η-C8H6{SiiPr3-1,4}2)(η-Cp*)]2(μ-η1:η1-C2O2). J. Am. Chem. Soc. 130, 13816–13817 (2008).
Arnold, P. L., Turner, Z. R., Bellabarba, R. M. & Tooze, R. P. Carbon monoxide coupling and functionalisation at a simple uranium coordination complex. Chem. Sci. 2, 77–79 (2011).
Miller, A. J. M., Labinger, J. A. & Bercaw, J. E. Reductive coupling of carbon monoxide in a rhenium carbonyl complex with pendant Lewis acids. J. Am. Chem. Soc. 130, 11874–11875 (2008).
Watanabe, T., Ishida, Y., Matsuo, T. & Kawaguchi, H. Reductive coupling of six carbon monoxides by a ditantalum hydride complex. J. Am. Chem. Soc. 131, 3474–3475 (2009).
Wang, X. et al. Room-temperature reaction of carbon monoxide with a stable diarylgermylene. J. Am. Chem. Soc. 131, 6912–6913 (2009).
Brown, Z. D. & Power, P. P. Mechanisms of reactions of open-shell, heavier group 14 derivatives with small molecules: n−π* back-bonding in isocyanide complexes, C–H activation under ambient conditions, CO coupling, and ancillary molecular interactions. Inorg. Chem. 52, 6248–6259 (2013).
Teichmann, J., Stock, H., Pritzkow, H. & Siebert, W. Carbon monoxide and isonitrile insertion into the B–B bond of five-membered cyclic organo-1,2-diboranes. Eur. J. Inorg. Chem. 459–463 (1998).
Sander, W., Bucher, G. & Wierlacher, S. Carbenes in matrices—spectroscopy, structure, and reactivity. Chem. Rev. 93, 1583–1621 (1993).
Lavallo, V., Canac, Y., Donnadieu, B., Schoeller, W. W. & Bertrand, G. CO fixation to stable acyclic and cyclic alkyl amino carbenes: stable amino ketenes with a small HOMO–LUMO gap. Angew. Chem. Int. Ed. 45, 3488–3491 (2006).
Sajid, M. et al. Facile carbon monoxide reduction at intramolecular frustrated phosphane/borane Lewis pair templates. Angew. Chem. Int. Ed. 52, 2243–2246 (2013).
Dobrovetsky, R. & Stephan, D. W. Stoichiometric metal-free reduction of CO in syn-gas. J. Am. Chem. Soc. 135, 4974–4977 (2013).
Asay, M. & Sekiguchi, A. Recent developments in the reactivity of stable disilynes. Bull. Chem. Soc. Jpn 85, 1245–1261 (2012).
Segawa, Y., Suzuki, Y., Yamashita, M. & Nozaki, K. Chemistry of boryllithium: synthesis, structure, and reactivity. J. Am. Chem. Soc. 130, 16069–16079 (2008).
Aramaki, Y. et al. Synthesis and characterization of B-heterocyclic π-radical and its reactivity as a boryl radical. J. Am. Chem. Soc. 134, 19989–19992 (2012).
Kinjo, R., Donnadieu, B., Celik, M. A., Frenking, G. & Bertrand, G. Synthesis and characterization of a neutral tricoordinate organoboron isoelectronic with amines. Science 333, 610–613 (2011).
Braunschweig, H. et al. Ambient-temperature isolation of a compound with a boron–boron triple bond. Science 336, 1420–1422 (2012).
Olmstead, M. M., Power, P. P., Weese, K. J. & Doedens, R. J. Isolation and X-ray crystal structure of the boron methylidenide ion [Mes2BCH2]− (Mes = 2,4,6-Me3C6H2)—a boron–carbon double bonded alkene analogue. J. Am. Chem. Soc. 109, 2541–2542 (1987).
Hoefelmeyer, J. D., Sole, S. & Gabbaï, F. P. Reactivity of the dimesityl-1,8-naphthalenediylborate anion: isolation of the borataalkene isomer and synthesis of 1,8-diborylnaphthalenes. Dalton Trans. 1254–1258 (2004).
Chiu, C. W. & Gabbaï, F. P. Structural changes accompanying the stepwise population of a B–C π bond. Angew. Chem. Int. Ed. 46, 6878–6881 (2007).
Wuckelt, J., Doring, M., Langer, P., Gorls, H. & Beckert, R. Lactam analogues of pentalene. A new one-pot synthesis of pyrrolo[3,2-b]pyrrole-2,5-diones deriving from pulvinic acid. Tetrahedron Lett. 38, 5269–5272 (1997).
Gaylord, M. C., Benedict, R. G., Hatfield, G. M. & Brady, L. R. Isolation of diphenyl-substituted tetronic acids from cultures of Paxillus atrotomentosus. J. Pharm. Sci. 59, 1420–1423 (1970).
Lunak, S., Vynuchal, J. & Hrdina, R. Geometry and absorption of diketo-pyrrolo-pyrrole isomers and their pi-isoelectronic furo-furanone analogues. J. Mol. Struct. 919, 239–245 (2009).
Wang, Y. et al. A stable neutral diborene containing a B=B double bond. J. Am. Chem. Soc. 129, 12412–12413 (2007).
Jacobsen, H. et al. Lewis acid properties of tris(pentafluorophenyl)borane. Structure and bonding in L-B(C6F5)3 complexes. Organometallics 18, 1724–1735 (1999).
Connelly, N. G. & Geiger, W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Enemaerke, R. J., Daasbjerg, K. & Skrydstrup, T. Is samarium diiodide an inner- or outer-sphere electron donating agent? Chem. Commun. 343–344 (1999).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Braunschweig, H., Radacki, K., Shang, R. & Tate, C. W. Reversible intramolecular coupling of the terminal borylene and a carbonyl ligand of [Cp(CO)2Mn=B-tBu]. Angew. Chem. Int. Ed. 52, 729–733 (2012).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. BR 1149/13-1).
Author information
Authors and Affiliations
Contributions
H.B. conceived and supervised the study. J.M., T.D., K.H. and W.C.E. performed the syntheses and spectroscopic studies. J.O.C.J.H., A.V. and A.K.P. performed the DFT computational studies. I.K. performed the EPR spectroscopic and cyclic voltammetry. T.K. performed the X-ray crystallographic measurements. R.D.D. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 948 kb)
Supplementary information
Crystallographic data for compound 2. (CIF 36 kb)
Supplementary information
Crystallographic data for compound 3. (CIF 26 kb)
Rights and permissions
About this article
Cite this article
Braunschweig, H., Dellermann, T., Dewhurst, R. et al. Metal-free binding and coupling of carbon monoxide at a boron–boron triple bond. Nature Chem 5, 1025–1028 (2013). https://doi.org/10.1038/nchem.1778
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1778
This article is cited by
-
Direct synthesis of oxalic acid via oxidative CO coupling mediated by a dinuclear hydroxycarbonylcobalt(III) complex
Nature Communications (2023)
-
A silylene-stabilized ditin(0) complex and its conversion to methylditin cation and distannavinylidene
Nature Communications (2023)
-
A silicon–carbonyl complex stable at room temperature
Nature Chemistry (2020)
-
Selective reduction and homologation of carbon monoxide by organometallic iron complexes
Nature Communications (2018)
-
Ambiphilic boron in 1,4,2,5-diazadiborinine
Nature Communications (2016)