Abstract
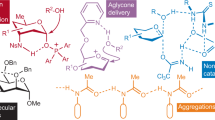
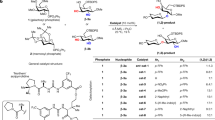
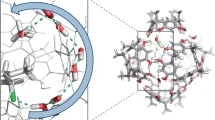
Carbohydrates and natural products serve essential roles in nature, and also provide core scaffolds for pharmaceutical agents and vaccines. However, the inherent complexity of these molecules imposes significant synthetic hurdles for their selective functionalization and derivatization. Nature has, in part, addressed these issues by employing enzymes that are able to orient and activate substrates within a chiral pocket, which increases dramatically both the rate and selectivity of organic transformations. In this article we show that similar proximity effects can be utilized in the context of synthetic catalysts to achieve general and predictable site-selective functionalization of complex molecules. Unlike enzymes, our catalysts apply a single reversible covalent bond to recognize and bind to specific functional group displays within substrates. By combining this unique binding selectivity and asymmetric catalysis, we are able to modify the less reactive axial positions within monosaccharides and natural products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mahatthananchai, J., Dumas, A. M. & Bode, J. W. Catalytic selective synthesis. Angew. Chem. Int. Ed. 51, 10954–10990 (2012).
Butler, M. S. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 25, 475–516 (2008).
van Kooyk, Y. & Rabinovich, G. A. Protein–glycan interactions in the control of innate and adaptive immune responses. Nature Immunol. 9, 593–601 (2008).
Helenius, A. & Aebi, M. Intracellular functions of N-linked glycans. Science 291, 2364–2369 (2001).
Weymouth-Wilson, A. C. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 14, 99–110 (1997).
La Ferla, B. et al. Natural glycoconjugates with antitumor activity. Nat. Prod. Rep. 28, 630–648 (2011).
Seeberger, P. H. & Werz, D. B. Automated synthesis of oligosaccharides as a basis for drug discovery. Nature Rev. Drug. Discov. 4, 751–763 (2005).
Zhu, X. & Schmidt, R. R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 48, 1900–1934 (2009).
Hsu, C. H., Hung, S. C., Wu, C. Y. & Wong, C. H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. 50, 11872–11923 (2011).
Walczak, M. A. & Danishefsky, S. J. Solving the convergence problem in the synthesis of triantennary N-glycan relevant to prostate-specific membrane antigen (PSMA). J. Am. Chem. Soc. 134, 16430–16433 (2012).
Breslow, R. et al. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 95, 3251–3262 (1973).
Breslow, R. et al. Selective halogenation of steroids using attached aryl iodide templates. J. Am. Chem. Soc. 99, 905–915 (1977).
Breslow, R. & Heyer, D. Catalytic multiple template-directed steroid chlorinations. J. Am. Chem. Soc. 104, 2045–2046 (1982).
Lewis, C. A. & Miller, S. J. Site-selective derivatization and remodeling of erythromycin A by using simple peptide-based chiral catalysts. Angew. Chem. Int. Ed. 45, 5616–5619 (2006).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Yoshida, K., Furuta, T. & Kawabata, T. Perfectly regioselective acylation of a cardiac glycoside, digitoxin, via catalytic amplification of the intrinsic reactivity. Tetrahedron Lett. 51, 4830–4832 (2010).
Snyder, S. A., Gollner, A. & Chiriac, M. I. Regioselective reactions for programmable resveratrol oligomer synthesis. Nature 474, 461–466 (2011).
Bruckl, T., Baxter, R. D., Ishihara, Y. & Baran, P. S. Innate and guided C–H functionalization logic. Acc. Chem. Res. 45, 826–839 (2012).
Pathak, T. P. & Miller, S. J. Site-selective bromination of vancomycin. J. Am. Chem. Soc. 134, 6120–6123 (2012).
Wilcock, B. C. et al. Electronic tuning of site-selectivity. Nature Chem. 4, 996–1003 (2012).
Fowler, B. S., Laemmerhold, K. M. & Miller, S. J. Catalytic site-selective thiocarbonylations and deoxygenations of vancomycin reveal hydroxyl-dependent conformational effects. J. Am. Chem. Soc. 134, 9755–9761 (2012).
Beale, T. M. & Taylor, M. S. Synthesis of cardiac glycoside analogs by catalyst-controlled, regioselective glycosylation of digitoxin. Org. Lett. 15, 1358–1361 (2013).
Pathak, T. P. & Miller, S. J. Chemical tailoring of teicoplanin with site-selective reactions. J. Am. Chem. Soc. 135, 8415–8422 (2013).
Lee, D. & Taylor, M. S. Catalyst-controlled regioselective reactions of carbohydrate derivatives. Synthesis 44, 3421–3431 (2012).
Kawabata, T., Muramatsu, W., Nishio, T., Shibata, T. & Schedel, H. A catalytic one-step process for the chemo- and regioselective acylation of monosaccharides. J. Am. Chem. Soc. 129, 12890–12895 (2007).
Kawabata, T. & Furuta, T. Nonenzymatic regioselective acylation of carbohydrates. Chem. Lett. 38, 640–647 (2009).
Griswold, K. S. & Miller, S. J. A peptide-based catalyst approach to regioselective functionalization of carbohydrates. Tetrahedron 59, 8869–8875 (2003).
Gouliaras, C., Lee, D., Chan, L. & Taylor, M. S. Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J. Am. Chem. Soc. 133, 13926–13929 (2011).
Chan, L. & Taylor, M. S. Regioselective alkylation of carbohydrate derivatives catalyzed by a diarylborinic acid derivative. Org. Lett. 13, 3090–3093 (2011).
Lee, D. & Taylor, M. S. Borinic acid-catalyzed regioselective acylation of carbohydrate derivatives. J. Am. Chem. Soc. 133, 3724–3727 (2011).
Lee, D., Williamson, C. L., Chan, L. & Taylor, M. S. Regioselective, borinic acid-catalyzed monoacylation, sulfonylation and alkylation of diols and carbohydrates: expansion of substrate scope and mechanistic studies. J. Am. Chem. Soc. 134, 8260–8267 (2012).
Hu, G. & Vasella, A. Regioselective benzoylation of 6-O-protected and 4,6-O-diprotected hexopyranosides as promoted by chiral and achiral ditertiary 1,2-diamines. Helv. Chim. Acta 85, 4369–4391 (2002).
Page, M. I. & Jencks, W. P. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and chelate effect. Proc. Natl Acad. Sci. USA 68, 1678–1683 (1971).
Lobsanov, Y. D. et al. Structure of Kre2p/Mnt1p: a yeast α 1,2-mannosyltransferase involved in mannoprotein biosynthesis. J. Biol. Chem. 279, 17921–17931 (2004).
Sun, X., Worthy, A. D. & Tan, K. L. Scaffolding catalysts: highly enantioselective desymmetrization reactions. Angew. Chem. Int. Ed. 50, 8167–8171 (2011).
Worthy, A. D., Sun, X. & Tan, K. L. Site-selective catalysis: toward a regiodivergent resolution of 1,2-diols. J. Am. Chem. Soc. 134, 7321–7324 (2012).
Zhao, Y., Rodrigo, J., Hoveyda, A. H. & Snapper, M. L. Enantioselective silyl protection of alcohols catalysed by an amino-acid-based small molecule. Nature 443, 67–70 (2006).
Isobe, T., Fukuda, K., Araki, Y. & Ishikawa, T. Modified guanidines as chiral superbases: the first example of asymmetric silylation of secondary alcohols. Chem. Commun. 7, 243–244 (2001).
Weickgenannt, A., Mewald, M. & Oestreich, M. Asymmetric Si–O coupling of alcohols. Org. Biomol. Chem. 8, 1497–1504 (2010).
Zhao, Y., Mitra, A. W., Hoveyda, A. H. & Snapper, M. L. Kinetic resolution of 1,2-diols through highly site- and enantioselective catalytic silylation. Angew. Chem. Int. Ed. 46, 8471–8474 (2007).
Rodrigo, J. M., Zhao, Y., Hoveyda, A. H. & Snapper, M. L. Regiodivergent reactions through catalytic enantioselective silylation of chiral diols. Synthesis of sapinofuranone A. Org. Lett. 13, 3778–3781 (2011).
Sheppard, C. I., Taylor, J. L. & Wiskur, S. L. Silylation-based kinetic resolution of monofunctional secondary alcohols. Org. Lett. 13, 3794–3797 (2011).
Weickgenannt, A., Mohr, J. & Oestreich, M. Catalytic enantioselective dehydrogenative Si–O coupling of oxime ether-functionalized alcohols. Tetrahedron 68, 3468–3479 (2012).
Pascal, R. Catalysis through induced intramolecularity: what can be learned by mimicking enzymes with carbonyl compounds that covalently bind substrates? Eur. J. Org. Chem., 10, 1813–1824 (2003).
Tan, K. L. Induced intramolecularity: an effective strategy in catalysis. ACS Cat. 1, 877–886 (2011).
Guimond, N., MacDonald, M. J., Lemieux, V. & Beauchemin, A. M. Catalysis through temporary intramolecularity: mechanistic investigations on aldehyde-catalyzed Cope-type hydroamination lead to the discovery of a more efficient tethering catalyst. J. Am. Chem. Soc. 134, 16571–16577 (2012).
MacDonald, M. J., Hesp, C. R., Schipper, D. J., Pesant, M. & Beauchemin, A. M. Highly enantioselective intermolecular hydroamination of allylic amines with chiral aldehydes as tethering catalysts. Chem. Eur. J. 19, 2597–2601 (2013).
Somoza, A. Protecting groups for RNA synthesis: an increasing need for selective preparative methods. Chem. Soc. Rev. 37, 2668–2675 (2008).
Repke, K. R. H. & Megges, R. Status and prospect of current inotropic agents. Expert Opin. Ther. Pat. 7, 1297–1306 (1997).
Thomas, C. M., Hothersall, J., Willis, C. L. & Simpson, T. J. Resistance to and synthesis of the antibiotic mupirocin. Nature Rev. Microbiol. 8, 281–289 (2010).
Silvian, L. F., Wang, J. & Steitz, T. A. Insights into editing from an Ile-tRNA synthetase structure with tRNA(Ile) and mupirocin. Science 285, 1074–1077 (1999).
Acknowledgements
This research was supported by the National Institutes of Health (RO1-GM087581), National Science Foundation Career Award (CHE-1150393) and Boston College. X.S. is an AstraZeneca Graduate Fellow and K.L.T. is an Alfred P. Sloan fellow. We thank P. Ozkal for early experimental assistance; E. Weerapana, J. Morken and A. Hoveyda for discussions; R. Jain, H. Pham and Novartis for providing spectra of α-acetyl digoxin.
Author information
Authors and Affiliations
Contributions
K.L.T. and X.S. were involved in the design and discovery of the catalysts; X.S. was responsible for the data obtained with methyl-α-D-mannose, arabinose, galactose and digoxin; H.L. was responsible for the data obtained with methyl-α-L-rhamnose and mupirocin; S.L. was responsible for the data obtained with uridine; K.L.T. conceived and directed the investigation and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4429 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Lee, H., Lee, S. et al. Catalyst recognition of cis-1,2-diols enables site-selective functionalization of complex molecules. Nature Chem 5, 790–795 (2013). https://doi.org/10.1038/nchem.1726
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1726
This article is cited by
-
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
Nature Chemistry (2023)
-
Site-selective, stereocontrolled glycosylation of minimally protected sugars
Nature (2022)
-
Exploiting non-covalent interactions in selective carbohydrate synthesis
Nature Reviews Chemistry (2021)
-
Site-switchable mono-O-allylation of polyols
Nature Communications (2020)
-
Site-selective oxidation, amination and epimerization reactions of complex polyols enabled by transfer hydrogenation
Nature Chemistry (2017)