Abstract
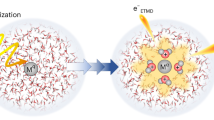
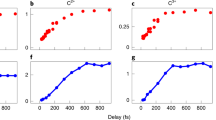
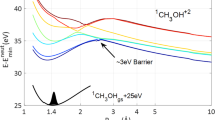
To understand the yield and patterns of damage in aqueous condensed matter, including biological systems, it is essential to identify the initial products subsequent to the interaction of high-energy radiation with liquid water. Until now, the observation of several fast reactions induced by energetic particles in water was not possible on their characteristic timescales. Therefore, some of the reaction intermediates involved, particularly those that require nuclear motion, were not considered when describing radiation chemistry. Here, through a combined experimental and theoretical study, we elucidate the ultrafast proton dynamics in the first few femtoseconds after X-ray core-level ionization of liquid water. We show through isotope analysis of the Auger spectra that proton-transfer dynamics occur on the same timescale as electron autoionization. Proton transfer leads to the formation of a Zundel-type intermediate [HO*···H···H2O]+, which further ionizes to form a so-far unnoticed type of dicationic charge-separated species with high internal energy. We call the process proton-transfer mediated charge separation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seiwert, T. Y., Salama, J. K. & Vokes, E. E. The concurrent chemoradiation paradigm – general principles. Nature Clin. Pract. Oncol. 4, 86–100 (2007).
Howell, R. W. Auger processes in the 21st century. Int. J. Radiat. Biol. 84, 959–975 (2008).
Eschenbrenner, A. et al. Strand breaks induced in plasmid DNA by ultrasoft X-rays: influence of hydration and packing. Int. J. Radiat. Biol. 83, 687–697 (2007).
Wang, C. R., Nguyen, J. & Lu, Q. B. Bond breaks of nucleotides by dissociative electron transfer of nonequilibrium prehydrated electrons: a new molecular mechanism for reductive DNA damage. J. Am. Chem. Soc. 131, 11320–11322 (2009).
Alizadeh, E. & Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 112, 5578–5602 (2012).
Weik, M. et al. Specific chemical and structural damage to proteins produced by synchrotron radiation. Proc. Natl Acad. Sci. USA 97, 623–628 (2000).
Hatano, Y., Katsumura, Y. & Mozumder, A. Charged Particle and Photon Interactions with Matter: Recent Advances, Applications, and Interfaces (CRC Press, 2010).
Garrett, B. C. et al. Role of water in electron-initiated processes and radical chemistry: issues and scientific advances. Chem. Rev. 105, 355–389 (2005).
Meesungnoen, J. et al. Multiple ionization effects on the yields of HO2/O2− and H2O2 produced in the radiolysis of liquid water with high-LET 12C6+ ions: a Monte-Carlo simulation study. Chem. Phys. Lett. 377, 419–425 (2003).
Tavernelli, I. et al. Time-dependent density functional theory molecular dynamics simulations of liquid water radiolysis. Chem. Phys. Chem. 9, 2099–2103 (2008).
Gaigeot, M. P. et al. A multi-scale ab initio theoretical study of the production of free radicals in swift ion tracks in liquid water. J. Phys. B 40, 1–12 (2007).
Cederbaum, L. S., Zobeley, J. & Tarantelli, F. Giant intermolecular decay and fragmentation of clusters. Phys. Rev. Lett. 79, 4778–4781 (1997).
Müller, I. B. & Cederbaum, L. S. Electronic decay following ionization of aqueous Li+ microsolvation clusters. J. Chem. Phys. 122, 194305 (2005).
Hergenhahn, U. Interatomic and intermolecular Coulombic decay: the early years. J. Electron Spectrosc. Relat. Phenom. 184, 78–90 (2011).
Marburger, S., Kugeler, O., Hergenhahn, U. & Möller, T. Experimental evidence for interatomic Coulombic decay in Ne clusters. Phys. Rev. Lett. 90, 203401 (2003).
Jahnke, T. et al. Experimental observation of interatomic Coulombic decay in neon dimers. Phys. Rev. Lett. 93, 163401 (2004).
Hergenhahn, U. Production of low kinetic energy electrons and energetic ion pairs by intermolecular Coulombic decay. Int. J. Radiat. Biol. 88, 871–883 (2012).
Mucke, M. et al. A hitherto unrecognized source of low-energy electrons in water. Nature Phys. 6, 78–81 (2010).
Schwartz, C. P., Fatehi, S., Saykally, R. J. & Prendergast, D. Importance of electronic relaxation for inter-Coulombic decay in aqueous systems. Phys. Rev. Lett. 105, 198102 (2010).
Lindblad, A. et al. Charge delocalization dynamics of ammonia in different hydrogen bonding environments: free clusters and in liquid water solution. Phys. Chem. Chem. Phys. 11, 1758–1764 (2009).
Stoychev, S. D., Kuleff, A. I. & Cederbaum, L. S. Intermolecular Coulombic decay in small biochemically relevant hydrogen-bonded systems. J. Am. Chem. Soc. 133, 6817–6824 (2011).
Aziz, E. F., Ottosson, N., Faubel, M., Hertel, I. V. & Winter, B. Interaction between liquid water and hydroxide revealed by core-hole de-excitation. Nature 455, 89–91 (2008).
Pokapanich, W. et al. Auger electron spectroscopy as a probe of the solution of aqueous ions. J. Am. Chem. Soc. 131, 7264–7271 (2009).
Pokapanich, W. et al. Ionic-charge dependence of tie intermolecular Coulombic decay time scale for aqueous ions probed by the core-hole clock. J. Am. Chem. Soc. 133, 13430–13436 (2011).
Pokapanich, W. et al. Bond breaking, electron pushing, and proton pulling: active and passive roles in the interaction between aqueous ions and water as manifested in the O1s Auger decay. J. Phys. Chem. B 116, 3–8 (2012).
Ottosson, N., Öhrwall, G. & Björneholm, O. Ultrafast charge delocalization dynamics in aqueous electrolytes: new insights from Auger electron spectroscopy. Chem. Phys. Lett. 543, 1–11 (2012).
Öhrwall, G. et al. The electronic structure of free water clusters probed by Auger electron spectroscopy. J. Chem. Phys. 123, 054310 (2005).
Stoychev, S. D., Kuleff, A. I. & Cederbaum, L. S. On the intermolecular Coulombic decay of singly and doubly ionized states of water dimer. J. Chem. Phys. 133, 154307 (2010).
Kryzhevoi, N. V. & Cederbaum, L. S. Non local effects in the core ionization and Auger spectra of small ammonia clusters. J. Phys. Chem. B 115, 5441–5447 (2011).
Hjelte, I. et al. Evidence for ultra-fast dissociation of molecular water from resonant Auger spectroscopy. Chem. Phys. Lett. 334, 151–158 (2001).
Björneholm, O., Nilsson, A., Sandell, A., Hernnas, B. & Martensson, N. Determination of time scales for charge-transfer screening in physisorbed molecules. Phys. Rev. Lett. 68, 1892–1895 (1992).
Schnadt, J. et al. Experimental evidence for sub-3-fs charge transfer from an aromatic adsorbate to a semiconductor. Nature 418, 620–623 (2002).
Föhlisch, A. et al. Direct observation of electron dynamics in the attosecond domain. Nature 436, 373–376 (2005).
Odelius, M. et al. Ultrafast core-hole-induced dynamics in water probed by X-ray emission spectroscopy. Phys. Rev. Lett. 94, 227401 (2005).
Fuchs, O. et al. Isotope and temperature effects in liquid water probed by X-ray absorption and resonant X-ray emission spectroscopy. Phys. Rev. Lett. 100, 027801 (2008).
Tokushima, T. et al. High resolution X-ray emission spectroscopy of liquid water: the observation of two structural motifs. Chem. Phys. Lett. 460, 387–400 (2008).
Odelius, M. Molecular dynamics simulations of fine structure in oxygen K-edge X-ray emission spectra of liquid water and ice. Phys. Rev. B 79, 144204 (2009).
Ljungberg, M. P., Nilsson, A. & Pettersson, L. G. M. Semiclassical description of nuclear dynamics in X-ray emission of water. Phys. Rev. B 82, 245115 (2010).
Ljungberg, M. P., Pettersson, L. G. M. & Nilsson, A. Vibrational interference effects in X-ray emission of a model water dimer: implications for the interpretation of the liquid spectrum. J. Chem. Phys. 134, 044513 (2011).
Winter, B., Aziz, E. F., Hergenhahn, U., Faubel, M. & Hertel, I. V. Hydrogen bonds in liquid water studied by photoelectron spectroscopy. J. Chem. Phys. 126, 124504 (2007).
Winter, B., Hergenhahn, U., Faubel, M., Björneholm, O. & Hertel, I. V. Hydrogen bonding in liquid water probed by resonant Auger-electron spectroscopy. J. Chem. Phys. 127, 094501 (2007).
Nordlund, D. et al. Probing the electron delocalization in liquid water and ice at attosecond time scales. Phys. Rev. Lett. 99, 217406 (2007).
Soper, A. K. & Benmore, C. J. Quantum differences between heavy and light water. Phys. Rev. Lett. 101, 065502 (2008).
Winter, B. et al. Full valence band photoemission from liquid water using EUV synchrotron radiation. J. Phys. Chem. A 108, 2625–2632 (2004).
Nishizawa, K. et al. High-resolution soft X-ray photoelectron spectroscopy of liquid water. Phys. Chem. Chem. Phys. 13, 413–417 (2011).
Barth, S. et al. Valence ionization of water clusters: from isolated molecules to bulk. J. Phys. Chem. A 113, 13519–13527 (2009).
Takahashi, O. et al. Auger decay calculations with core-hole excited-state molecular-dynamics simulations of water. J. Chem. Phys. 124, 064307 (2006).
Felicissimo, V. C., Guimaraes, F. F., Gel'mukhanov, F., Cesar, A. & Ågren, H. The principles of infrared-X-ray pump-probe spectroscopy. Applications on proton transfer in core-ionized water dimers. J. Chem. Phys. 122, 094319 (2005).
Felicissimo, V. C. et al. A theoretical study of the role of the hydrogen bond on core ionization of the water dimer. Chem. Phys. 312, 311–318 (2005).
Stia, C. R. et al. Theoretical investigation of the ultrafast dissociation of core-ionized water and uracil molecules immersed in liquid water. Eur. Phys. J. D 60, 77–83 (2010).
Vendrell, O., Stoychev, S. D. & Cederbaum, L. S. Generation of highly damaging H2O+ radicals by inner valence shell ionization of water. Chem. Phys. Chem. 11, 1006–1009 (2010).
Marsalek, O. et al. Chasing charge localization and chemical reactivity following photoionization in liquid water. J. Chem. Phys. 135, 224510 (2011).
Anicich, V. G. Evaluated biomolecular ion-molecule gas-phase kinetics of positive ion for use in modeling planetary atmosphere, cometary comae, and interstellar clouds. J. Phys. Chem. Ref. Data 22, 1469–1569 (1993).
Pimblott, S. M. & LaVerne, J. A. Stochastic simulation of the electron radiolysis of water and aqueous solutions. J. Phys. Chem. A 101, 5828–5838 (1997).
Garrett, B. C. Ions at the air/water interface. Science 303, 1146–1147 (2004).
Jahnke, T. et al. Ultrafast energy transfer between water molecules. Nature Phys. 6, 139–142 (2010).
Winter, B. & Faubel, M. Photoemission from liquid aqueous solutions. Chem. Rev. 106, 1176–1211 (2006).
Winter, B. Liquid microjet for photoelectron spectroscopy. Nucl. Instrum. Meth. A 601, 139–150 (2009).
Seidel, R., Thürmer, S. & Winter, B. Photoelectron spectroscopy meets aqueous solution: studies from a vacuum liquid microjet. J. Phys. Chem. Lett. 2, 633–641 (2011).
MOLPRO, version 2010.1. A package of ab initio programs (University College, Cardiff, 2010).
Acknowledgements
We thank N. Kryzhevoi and L. S. Cederbaum for stimulating discussions. We acknowledge the support of the Grant agency of the Czech Republic via grants no. P208/10/1724 and P208/11/0161 to P.S., the US National Science Foundation (CHE-0957869) to S.E.B., the Deutsche Forschungsgemeinschaft (DFG) via projects WI 1327/3-1, UH 3060/5-1, and the DFG Research Unit FOR 1789.
Author information
Authors and Affiliations
Contributions
S.T. and B.W. conceived, designed and performed the experiments, and analysed the data. P.S. and M.O. conducted the calculations and contributed to data interpretation. N.O. and R.S. contributed materials and/or analysis tools. B.W., P.S., S.T., U.H. and S.E.B. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Thürmer, S., Ončák, M., Ottosson, N. et al. On the nature and origin of dicationic, charge-separated species formed in liquid water on X-ray irradiation. Nature Chem 5, 590–596 (2013). https://doi.org/10.1038/nchem.1680
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1680
This article is cited by
-
Experimental quantification of site-specific efficiency of Interatomic Coulombic Decay after inner shell ionization
Communications Physics (2023)
-
H2 formation via non-Born-Oppenheimer hydrogen migration in photoionized ethane
Nature Communications (2023)
-
Radiation damage by extensive local water ionization from two-step electron-transfer-mediated decay of solvated ions
Nature Chemistry (2023)
-
Ultrafast energy transfer between π-stacked aromatic rings upon inner-valence ionization
Nature Chemistry (2022)
-
Ultrafast structural rearrangement dynamics induced by the photodetachment of phenoxide in aqueous solution
Nature Communications (2019)