Abstract
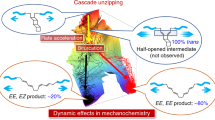
Recent force microscopy measurements on the mechanically activated cleavage of a protein disulfide bond through reaction with hydroxide ions revealed that for forces greater than 0.5 nN, the acceleration of the reaction rate is substantially reduced. Here, using ab initio simulations, we trace this ‘reactivity switch’ back to a dual role played by the mechanical force, which leads to antagonistic effects. On the one hand, the force performs work on the system, and thereby accelerates the reaction. On the other hand, the force also induces a conformational distortion that involves the S–S–C–C dihedral angle, which drives the disulfide into a conformation that is shielded against nucleophilic attack because of steric hindrance. The discovery of force-induced conformational changes that steer chemical reactivity provides a new key concept that is expected to be relevant beyond this specific case, for example in understanding how ‘disulfide switches’ regulate protein function and for the rational design of mechanoresponsive materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beyer, M. K. & Clausen-Schaumann, H. Mechanochemistry: the mechanical activation of covalent bonds. Chem. Rev. 105, 2921–2948 (2005).
Caruso, M. M. et al. Mechanically induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Black, A. L., Lenhardt, J. M. & Craig, S. L. From molecular mechanochemistry to stress-responsive materials. J. Mater. Chem. 21, 1655–1663 (2011).
Ribas-Arino, J. & Marx, D. Covalent mechanochemistry: theoretical concepts and computational tools with applications to molecular nanomechanics. Chem. Rev. 112, 5412–5487 (2012).
Wiita, A. P., Ainavarapu, S. R. K., Huang, H. H. & Fernandez, J. M. Force-dependent chemical kinetics of disulfide bond reduction observed with single-molecule techniques. Proc. Natl Acad. Sci. USA 103, 7222–7227 (2006).
Wiita, A. P. et al. Probing the chemistry of thioredoxin catalysis with force. Nature 450, 124–127 (2007).
Ainavarapu, S. R. K., Wiita, A. P., Dougan, L., Uggerud, E. & Fernandez, J. M. Single-molecule force spectroscopy measurements of bond elongation during a bimolecular reaction. J. Am. Chem. Soc. 130, 6479–6487 (2008).
Liang, J. & Fernandez, J. M. Mechanochemistry: one bond at a time. ACSNano 3, 1628–1645 (2009).
Garcia-Manyes, S., Liang, J., Szoszkiewicz, R., Kuo, T. L. & Fernandez, J. M. Force-activated reactivity switch in a bimolecular chemical reaction. Nature Chem. 1, 236–242 (2009).
Kucharski, T. J. et al. Kinetics of thiol/disulfide exchange correlate weakly with the restoring force in the disulfide moiety. Angew. Chem. Int. Ed. 48, 7040–7043 (2009).
Liang, J. & Fernandez, J. M. Kinetic measurements on single-molecule disulfide bond cleavage. J. Am. Chem. Soc. 133, 3528–3534 (2011).
Alegre-Cebollada, J., Kosuri, P., Rivas-Pardo, J. A. & Fernandez, J. M. Direct observation of disulfide isomerization in a single protein. Nature Chem. 3, 882–887 (2011).
Wouters, M. A., Fan, S. W. & Haworth, N. L. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid. Redox Signal. 12, 53–91 (2010).
Matthias, L. J. et al. Disulfide exchange in domain 2 of CD4 is required for entry of HIV-I. Nature Immunol. 3, 727–732 (2002).
Ahamed, J. et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc. Natl Acad. Sci. USA 103, 13932–13937 (2006).
Huang, Z. & Boulatov, R. Chemomechanics: chemical kinetics for multiscale phenomena. Chem. Soc. Rev. 40, 2359–2384 (2011).
Hofbauer, F. & Frank, I. Disulfide bond cleavage: a redox reaction without electron transfer. Chem. Eur. J. 16, 5097–5101 (2010).
Li, W. & Gräter, F. Atomistic evidence of how force dynamically regulates thiol/disulfide exchange. J. Am. Chem. Soc. 132, 16790–16795 (2010).
Iozzi, M. F., Helgaker, T. & Uggerud, E. Influence of external force on properties and reactivity of disulfide bonds. J. Phys. Chem. A 115, 2308–2315 (2011).
Baldus, I. B. & Gräter, F. Mechanical force can fine-tune redox potentials of disulfide bonds. Biophys. J. 102, 622–629 (2012).
Keten, S., Chou, C-C., van Duin, A. C. & Buehler, M. J. Tunable nanomechanics of protein disulfide bonds in redox microenvironments. J. Mech. Behav. Biomed. Mater. 5, 32–40 (2012).
Hofbauer, F. & Frank, I. CPMD simulation of a bimolecular chemical reaction: nucleophilic attack of a disulfide bond under mechanical stress. Chem. Eur. J. 18, 16332–16338 (2012).
Pappas, J. A. Theoretical studies of the reactions of the sulfur–sulfur bond. 1. General heterolytic mechanisms. J. Am. Chem. Soc. 99, 2926–2930 (1977).
Bachrach, S. M. & Mulhearn, D. C. Nucleophilic substitution at sulfur: SN2 or addition–elimination? J. Phys. Chem. 100, 3535–3540 (1996).
Fernandes, P. A. & Ramos, M. J. Theoretical insights into the mechanism for thiol/disulfide exchange. Chem. Eur. J. 10, 257–266 (2004).
Dmitrenko, O., Thorpe, C. & Bach., R. Mechanism of SN2 disulfide bond cleavage by phosphorus nucleophiles. Implications for biochemical disulfide reducing agents. J. Org. Chem. 72, 8298–8307 (2007).
Bach, R. D., Dmitrenko, O. & Thorpe, C. Mechanism of thiolate–disulfide interchange reactions in biochemistry. J. Org. Chem. 73, 12–21 (2008).
Marx, D. & Hutter, J. Ab Initio Molecular Dynamics: Basic Theory and Advanced Methods (Cambridge University Press, 2009).
Hayes, J. M. & Bachrach, S. M. Effect of micro and bulk solvation on the mechanism of nucleophilic substitution at sulfur in disulfides. J. Phys. Chem. A 107, 7952–7961 (2003).
Laio, A. & Parrinello, M. Escaping free-energy minima. Proc. Natl Acad. Sci. USA 99, 12562–12566 (2002).
Iannuzzi, M., Laio, A. & Parrinello, M. Efficient exploration of reactive potential energy surfaces using Car–Parrinello molecular dynamics. Phys. Rev. Lett. 90, 238302 (2003).
Carter, E., Ciccotti, G., Hynes, J. T. & Kapral, R. Constrained reaction coordinate dynamics for the simulation of rare events. Chem. Phys. Lett. 156, 472–477 (1989).
Sprik, M. & Ciccotti, G. Free energy from constrained molecular dynamics. J. Chem. Phys. 109, 7737–7744 (1998).
Dopieralski, P., Ribas-Arino, J. & Marx, D. Force-transformed free energy surfaces and trajectory shooting simulations reveal the mechano-stereochemistry of cyclopropane ring-opening reactions. Angew. Chem. Int. Ed. 50, 7105–7108 (2011).
Ribas-Arino, J., Shiga, M. & Marx, D. Understanding covalent mechanochemistry. Angew. Chem. Int. Ed. 48, 4190–4193 (2009).
Singh, R. & Whitesides, G. M. in Supplement S: The Chemistry of Sulphur-Containing Functional Groups (eds Patai, S. & Rappoport, Z.) 633–658 (John Wiley, 1993).
Marx, D., Chandra, A. & Tuckerman, M. E. Aqueous basic solutions: hydroxide solvation, structural diffusion, and comparison to the hydrated proton. Chem. Rev. 110, 2174–2216 (2010).
Hickenboth, C. R. et al. Biasing reaction pathways with mechanical force. Nature 446, 423–427 (2007).
Ribas-Arino, J., Shiga, M. & Marx, D. Mechanochemical transduction of externally applied forces to mechanophores. J. Am. Chem. Soc. 132, 10609–10614 (2010).
Dopieralski, P. et al. On the role of polymer chains in transducing external mechanical forces to benzocyclobutene mechanophores. J. Mater. Chem. 21, 8309–8316 (2011).
Tian, Y. & Boulatov, R. Quantum-chemical validation of the local assumption of chemomechanics for a unimolecular reaction. ChemPhysChem 13, 2277–2281 (2012).
Haworth, N. L., Gready, J. E., George, R. A. & Wouters, M. A. Evaluating the stability of disulfide bridges in proteins: a torsional potential energy surface for diethyl disulfide. Mol. Simul. 33, 475–485 (2007).
Kuo, T-L. et al. Probing static disorder in Arrhenius kinetics by single-molecule spectroscopy. Proc. Natl Acad. Sci. USA 107, 11336–11340 (2010).
Garcia-Manyes, S., Kuo, T-L. & Fernandez, J. M. Contrasting the individual reactive pathways in protein unfolding and disulfide bond reduction observed within a single protein. J. Am. Chem. Soc. 133, 3104–3113 (2011).
Bailey, A. & Mosey, N. J. Prediction of reaction barriers and force-induced instabilities under mechanochemical conditions with an approximate model: a case study of the ring opening of 1,3-cyclohexadiene. J. Chem. Phys. 136, 044102 (2012).
Marshall, B. T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003).
Prezhdo, O. V. & Pereverzev, Y. V. Theoretical aspects of the biological catch bond. Acc. Chem. Res. 42, 693–703 (2009).
Acknowledgements
We are grateful to the Deutsche Forschungsgemeinsschaft (Reinhart Koselleck Grant Understanding Mechanochemistry (MA 1547/9) and Cluster of Excellence RESOLV (EXC 1069)), Alexander von Humboldt Stiftung (Humboldt Fellowship to J.R-A), the Spanish Government (Ramón y Cajal Fellowship to J.R-A.) and the Research Department Interfacial Systems Chemistry for partial financial support. The authors gratefully acknowledge the Gauss Centre for Supercomputing (GCS) for providing computing time for a GCS Large Scale Project on JUQUEEN at Jülich Supercomputing Centre (JSC) as well as computational support by BOVILAB@RUB, Rechnerverbund-NRW, Wroclaw Supercomputer Center (WCSS), Galera-ACTION Cluster and Academic Computer Center in Gdańsk (CI TASK).
Author information
Authors and Affiliations
Contributions
P.D, J.R-A. and D.M. conceived and designed the research; P.D. performed the calculations; P.D, J.R-A., P.A., M.K. and D.M. analysed the data; J.K. contributed analysis tools; J.R-A., P.D. and D.M. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1557 kb)
Rights and permissions
About this article
Cite this article
Dopieralski, P., Ribas-Arino, J., Anjukandi, P. et al. The Janus-faced role of external forces in mechanochemical disulfide bond cleavage. Nature Chem 5, 685–691 (2013). https://doi.org/10.1038/nchem.1676
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1676
This article is cited by
-
Mechanochemical bond scission for the activation of drugs
Nature Chemistry (2021)
-
Sterically controlled mechanochemistry under hydrostatic pressure
Nature (2018)
-
Forcing the reversibility of a mechanochemical reaction
Nature Communications (2018)
-
Unexpected mechanochemical complexity in the mechanistic scenarios of disulfide bond reduction in alkaline solution
Nature Chemistry (2017)
-
Tailoring protein nanomechanics with chemical reactivity
Nature Communications (2017)