Abstract
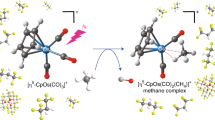
The controlled cleavage of strong C–H bonds such as those of methane poses a significant and industrially important challenge for chemists. In nature, methane is oxidized to methanol by soluble methane monooxygenase via a diiron(iv) intermediate called Q. However, the only two reported diiron(iv) complexes have activities towards C–H bonds that fall far short of the activity of this biological catalyst. In this paper, we model the chemistry of MMO-Q by generating an oxo-bridged diiron(iv) complex by electrochemical oxidation. This species is a more effective oxidant. It can attack C–H bonds as strong as 100 kcal mol−1 and reacts with cyclohexane 100- to 1,000-fold faster than mononuclear FeIV=O complexes of closely related ligands. Strikingly, this species can also cleave the strong O–H bonds of methanol and t-butanol instead of their weaker C–H bonds, representing the first example of O–H bond activation for iron complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanson, R. S. & Hanson, T. E. Methanotrophic bacteria. Microbiol. Rev. 60, 439–471 (1996).
Wallar, B. J. & Lipscomb, J. D. Dioxygen activation by enzymes containing binuclear non-heme iron clusters. Chem. Rev. 96, 2625–2657 (1996).
Merkx, M. et al. Dioxygen Activation and methane hydroxylation by soluble methane monooxygenase: A tale of two irons and three proteins. Angew. Chem. Int. Ed. 40, 2782–2807 (2001).
Balasubramanian, R. & Rosenzweig, A. C. Structural and mechanistic insights into methane oxidation by particulate methane monooxygenase. Acc. Chem. Res. 40, 573–580 (2007).
Lee, S.-K., Fox, B. G., Froland, W. A., Lipscomb, J. D. & Münck, E. A transient intermediate of the methane monooxygenase catalytic cycle containing an FeIVFeIV cluster. J. Am. Chem. Soc. 115, 6450–6451 (1993).
Liu, K. E. et al. Kinetic and spectroscopic characterization of intermediates and component interactions in reactions of methane monooxygenase from Methylococcus capsulatus (Bath). J. Am. Chem. Soc. 117, 10174–10185 (1995).
Nesheim, J. C. & Lipscomb, J. D. Large kinetic isotope effects in methane oxidation catalyzed by methane monooxygenase: Evidence for C–H bond cleavage in a reaction cycle intermediate. Biochemistry 35, 10240–10247 (1996).
Shu, L. et al. An FeIV2O2 diamond core structure for the key intermediate Q of methane monooxygenase. Science 275, 515–518 (1997).
Ghosh, A. et al. Catalytically active μ-oxodiiron(IV) oxidants from iron(III) and dioxygen. J. Am. Chem. Soc. 127, 2505–2513 (2005).
Xue, G. et al. A synthetic precedent for the [FeIV2(μ-O)2] diamond core proposed for methane monooxygenase intermediate Q. Proc. Natl Acad. Sci. USA 104, 20713–20718 (2007).
Xu, J.-Y. et al. Iron(III) complexes with the ligand N′,N′-bis[(2-pyridyl)-methyl]ethylenediamine (uns-penp) and its amide derivative N-acetyl-N′,N′-bis[(2-pyridyl)methyl]ethylenediamine (acetyl-uns-penp). Eur. J. Inorg. Chem. 1601–1610 (2006).
Kurtz, D. M. Jr Oxo- and hydroxo-bridged diiron complexes: A chemical perspective on a biological unit. Chem. Rev. 90, 585–606 (1990).
Slep, L. D. et al. Mixed-valent {FeIV(μ-O)(μ-carboxylato)2FeIII}3+ core. J. Am. Chem. Soc. 125, 15554–15570 (2003).
Norman, R. E. et al. (μ-Oxo)(μ-carboxylato)diiron(III) complexes with distinct iron sites. Consequences of the inequivalence and its relevance to dinuclear iron–oxo proteins. J. Am. Chem. Soc. 112, 1554–1562(1990).
Jackson, T. A. et al. Axial ligand effects on the geometric and electronic structures of nonheme oxoiron(IV) complexes. J. Am. Chem. Soc. 130, 12394–12407 (2008).
Rohde, J. U. et al. Nonheme oxoiron(IV) complexes of tris(2-pyridylmethyl)amine with cis-monoanionic ligands. Inorg. Chem. 45, 6435–6445 (2006).
Collins, M. J., Ray, K. & Que, L. Jr Electrochemical generation of a nonheme oxoiron(IV) complex. Inorg. Chem. 45, 8009–8011 (2006).
Kaizer, J. et al. Nonheme FeIVO complexes that can oxidize the C–H bonds of cyclohexane at room temperature. J. Am. Chem. Soc. 126, 472–473 (2004).
Kumar, D., de Visser, S. P., Sharma, P. K., Cohen, S. & Shaik, S. Radical clock substrates, their C–H hydroxylation mechanism by cytochrome P450, and other reactivity patterns: What does theory reveal about the clocks' behaviour? J. Am. Chem. Soc. 126, 1907–1920 (2004).
Kumar, D., de Visser, S. P. & Shaik, S. Oxygen economy of cytochrome P450: What is the origin of the mixed functionality as a dehydrogenase-oxidase enzyme compared with its normal function? J. Am. Chem. Soc. 126, 5072–5073 (2004).
Jin, Y. & Lipscomb, J. D. Desaturation reactions catalyzed by soluble methane monooxygenase. J. Biol. Inorg. Chem. 6, 717–725 (2001).
Griller, D. & Ingold, K. U. Free-radical clocks. Acc. Chem. Res. 13, 317–323 (1980).
Vollhardt, K. P. C. The reactions of alcohols and the chemistry of ethers, in Organic Chemistry 308–358 (W. H. Freeman, 1987).
Luo, Y.-R. in Comprehensive Handbook of Chemical Bond Energies 255–369 (Taylor & Francis, 2007).
Pestovsky, O. & Bakac, A. Reactivity of aqueous fe(IV) in hydride and hydrogen atom transfer reactions. J. Am. Chem. Soc. 126, 13757–13764 (2004).
Pestovsky, O. et al. Aqueous : Spectroscopic identification and oxo group exchange. Angew. Chem. Int. Ed. 44, 6871–6874 (2005).
Jacobsen, F., Holcman, J. & Sehested, K. Reactions of the ferryl ion with some compounds found in cloud water. Int. J. Chem. Kinet. 30, 215–221 (1998).
Shaik, S., Hirao, H. & Kumar, D. Reactivity of high-valent iron–oxo species in enzymes and synthetic reagents: A tale of many states. Acc. Chem. Res. 40, 532–542 (2007).
Sastri, C. V. et al. Axial ligand tuning of a nonheme iron(IV)–oxo unit for hydrogen atom abstraction. Proc. Natl Acad. Sci. USA 104, 19181–19186 (2007).
Decker, A. et al. Spectroscopic and quantum chemical studies on low-spin complexes: Fe–O bonding and its contributions to reactivity. J. Am. Chem. Soc. 129, 15983–15996 (2007).
Bernasconi, L., Louwerse, M. J. & Baerends, E. J. The role of equatorial and axial ligands in promoting the activity of non-heme oxidoiron(IV) catalysts in alkane hydroxylation. Eur. J. Inorg. Chem. 3023–3033 (2007).
Klinker, E. J. High-valent iron compounds supported by pentadentate ligands. PhD thesis, Univ. Minnesota–Twin Cities (2007).
Berben, L. A. & Peters, J. C. Dimanganese and diiron complexes of a binucleating cyclam ligand: Four-electron, reversible oxidation chemistry at high potentials. Inorg. Chem. 47, 11669–11679 (2008).
Berry, J. F., Bill, E., Bothe, E., Neese, F. & Wieghardt, K. Octahedral non-heme oxo and non-oxo Fe(IV) complexes: An experimental/theoretical comparison. J. Am. Chem. Soc. 128, 13515–13528 (2006).
Zheng, H., Yoo, S. J., Münck, E. & Que, L. Jr The flexible Fe2(μ-O)2 diamond core: A terminal iron(IV)–oxo species generated from the oxidation of a bis(μ-oxo)diiron(III) complex. J. Am. Chem. Soc. 122, 3789–3790 (2000).
Brazeau, B. J. & Lipscomb, J. D. Kinetics and thermodynamics of methane monooxygenase compound Q formation and reaction with substrates. Biochemistry 39, 13503–13515 (2000).
Valentine, A. M., Stahl, S. S. & Lippard, S. J. Mechanistic studies of the reaction of reduced methane monooxgyenase hydroxylase with dioxygen and substrates. J. Am. Chem. Soc. 121, 3876–3887 (1999).
Fox, B. G., Lyle, K. S. & Rogge, C. E. Reactions of the diiron enzyme stearoyl-acyl carrier protein desaturase. Acc. Chem. Res. 37, 421–429 (2004).
Luo, Y.-R. in Comprehensive Handbook of Chemical Bond Energies 19–147 (Taylor & Francis, 2007).
Acknowledgements
This work was supported by NIH Grants EB-001475 (E.M.) and GM-38767 (L.Q.) and NIH Graduate Traineeship GM-08700 (E.R.F.). XAS data were collected on beamlines 7-3 and 9-3 at the Stanford Synchrotron Radiation Lightsource (SSRL), a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Centre for Research Resources and the Biomedical Technology Program. We thank M. Latimer and A. Aranda for technical support and advice during XAS data collection. We also thank V.G. Young Jr and the X-ray Crystallographic Laboratory of the University of Minnesota for expert assistance in the crystal structure determination.
Author information
Authors and Affiliations
Contributions
D.W., E.M. and L.Q. conceived and designed the experiments; D.W., E.R.F. and A.S. performed the experiments; D.W., E.R.F., A.S. and E.M. analysed the data; D.W., E.R.F., A.S., E.M. and L.Q. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Supplementary information
Supplementary information
Supplementary information (PDF 738 kb)
Supplementary information
Crystallographic data for compound 1 (CIF 55 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Farquhar, E., Stubna, A. et al. A diiron(iv) complex that cleaves strong C–H and O–H bonds. Nature Chem 1, 145–150 (2009). https://doi.org/10.1038/nchem.162
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.162
This article is cited by
-
Proton-Coupled Electron Transfer in Organic Synthesis: Fundamentals, Applications, and Opportunities
Topics in Current Chemistry (2016)
-
Computational investigation on the catalytic activity of Rh6 and Rh4Ru2 clusters towards methanol activation
Theoretical Chemistry Accounts (2015)
-
An N-bridged high-valent diiron–oxo species on a porphyrin platform that can oxidize methane
Nature Chemistry (2012)
-
Diverting non-haem iron catalysed aliphatic C–H hydroxylations towards desaturations
Nature Chemistry (2011)
-
Million-fold activation of the [Fe2(µ-O)2] diamond core for C–H bond cleavage
Nature Chemistry (2010)