Abstract
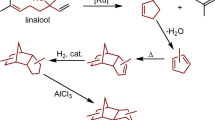
The conversion of biomass into fuels and chemical feedstocks is one part of a drive to reduce the world's dependence on crude oil. For transportation fuels in particular, wholesale replacement of a fuel is logistically problematic, not least because of the infrastructure that is already in place. Here, we describe the catalytic defunctionalization of a series of biomass-derived molecules to provide linear alkanes suitable for use as transportation fuels. These biomass-derived molecules contain a variety of functional groups, including olefins, furan rings and carbonyl groups. We describe the removal of these in either a stepwise process or a one-pot process using common reagents and catalysts under mild reaction conditions to provide n-alkanes in good yields and with high selectivities. Our general synthetic approach is applicable to a range of precursors with different carbon content (chain length). This allows the selective generation of linear alkanes with carbon chain lengths between eight and sixteen carbons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
26 April 2013
03 May 2013
In the version of this Article originally published, references to certain compound numbers in the Methods section were incorrect: Under the heading 'General experimental' "...conversion of 3 into n-nonane..." should have read "...conversion of 2 into n-nonane...". In 'Conversion of A into 2,5,8-nonanetrione 4', "... solvent removed in vacuo to yield 3..." should have read "... solvent removed in vacuo to yield 4...". In 'Conversion of B into 2,5,8-nonanetrione 4', "... solvent removed in vacuo to yield 3..." should have read "... solvent removed in vacuo to yield 4...". These errors have been corrected in the HTML and PDF versions of the Article.
References
Simmons, M. R. Twilight in the Desert: The Coming Saudi Oil Shock and the World Economy (Wiley, 2006).
Armaroli, N. & Balzani, V. The future of energy supply: challenges and opportunities. Angew. Chem. Int. Ed. 46, 52–66 (2007).
Kerr, R. A. Technology is turning US oil around but not the world's. Science 335, 522–523 (2012).
Murray, J. & King, D. Climate policy: oil's tipping point has passed. Nature 481, 433–435 (2012).
Tverberg, G. E. Oil supply limits and the continuing financial crisis. Energy 37, 27–34 (2012).
Exxon Mobil. Outlook for Energy for 2040. http://www.exxonmobil.com/Corporate/energy_outlook_view.aspx
IPCC. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. http://www.ipcc.ch/publications_and_data/ar4/syr/en/contents.html (2007).
Xing, R. et al. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 12, 1933–1946 (2010).
Schlaf, M. Selective deoxygenation of sugar polyols to [α],[ω]-diols and other oxygen content reduced materials—a new challenge to homogeneous ionic hydrogenation and hydrogenolysis catalysis. Dalton Trans. 4645–4653 (2006).
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006).
Keith, J. M. et al. Aqueous organocatalysis for the carbon chain extension of carbohydrate derivatives: application to the production of transportation fuels. Curr. Org. Chem. in the press.
Mascal, M. & Nikitin, E. B. Co-processing of carbohydrates and lipids in oil crops to produce a hybrid biodiesel. Energy & Fuels 24, 2170–2171 (2010).
Huber, G. W., Chheda, J. N., Barrett, C. J. & Dumesic, J. A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 308, 1446–1450 (2005).
Silks, L. A. et al. Method of carbon chain extension using novel aldol reaction. US patent application 20110040110 (17 August 2009).
Sutton, A. D. et al. Compounds and methods for the production of long chain hydrocarbons from biological sources. International patent application PCT/US2012/055340 (14 September 2012).
Gliński, M., Kijeński, J. & Jakubowski, A. Ketones from monocarboxylic acids: catalytic ketonization over oxide systems. Appl. Catal. A 128, 209–217 (1995).
Ram, S. & Spicer, L. D. Catalyst type and concentration dependence in catalytic transfer hydrogenolysis of α,β-unsaturated carbonyls and nitriles via ammonium formate. Synth. Commun. 22, 2683–2690 (1992).
Copes, J. P. & McKinkey, C. Production of 1,4-butanediol from tetrahydrofuran. US patent 2,686,817 (1954).
Watson, J. M. Butane-1,4-diol from hydrolytic reduction of furan. Product R&D 12, 310–311 (1973).
Di Mondo, D. et al. Stainless steel as a catalyst for the total deoxygenation of glycerol and levulinic acid in aqueous acidic medium. ACS Catal. 1, 355–364 (2011).
Waidmann, C. R. et al. Catalytic ring opening of furan rings within biomass derived substrates: an experimental and theoretical study. Catal. Sci. Technol. http://dx.doi.org/10.1039/C2CY20395B (2012).
Bissot, T. C. Surface impregnated catalyst. US patent 4,048,096 (1997).
Han, Y. F., Kumar, D., Sivadinarayana, C. & Goodman, D. W. Kinetics of ethylene combustion in the synthesis of vinyl acetate over a Pd/SiO2 catalyst. J. Catal. 224, 60–68 (2004).
Acknowledgements
The authors acknowledge financial support from the Laboratory Research and Development (LDRD) program at Los Alamos National Laboratory.
Author information
Authors and Affiliations
Contributions
A.D.S. made the initial discovery. A.D.S., M.S., L.A.S. and J.C.G. conceived and designed the experiments. A.D.S. and F.D.W. performed the experiments. R.W. and L.A.S. contributed materials. A.D.S. and J.C.G. co-wrote the paper. All authors discussed the results and provided input for the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1408 kb)
Rights and permissions
About this article
Cite this article
Sutton, A., Waldie, F., Wu, R. et al. The hydrodeoxygenation of bioderived furans into alkanes. Nature Chem 5, 428–432 (2013). https://doi.org/10.1038/nchem.1609
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1609
This article is cited by
-
One-pot synthesis of fuel precursor from acetoin fermentation broth using ionic liquid-based salting-out extraction system
Biotechnology for Biofuels and Bioproducts (2023)
-
Electrochemical carbonyl reduction on single-site M–N–C catalysts
Communications Chemistry (2023)
-
Subnanometric Cu clusters on atomically Fe-doped MoO2 for furfural upgrading to aviation biofuels
Nature Communications (2022)
-
Sustainable production of benzene from lignin
Nature Communications (2021)
-
The long-term performance of reconstructed MgAl hydrotalcite in the aldol condensation of furfural and acetone
Reaction Kinetics, Mechanisms and Catalysis (2021)