Abstract
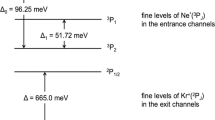
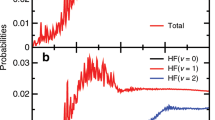
Elementary three-atom systems provide stringent tests of the accuracy of ab initio theory. One such important reaction, O(3P) + H2 → OH(X2Π) + H, has eluded detailed experimental study because of its high activation barrier. In this reaction, both the ground-state reactant atom and product diatomic molecule have open-shell character, which introduces the intriguing complication of non-Born–Oppenheimer effects in both the entrance and the exit channels. These effects may be probed experimentally in both the fine-structure and the Λ-doublet splittings of the OH product. We have used laser-induced fluorescence to measure OD internal product-state distributions from the analogous reaction of O(3P) with D2, enabled by a unique high-energy O(3P) source. We find that the OD (ν′ = 0) product is rotationally highly excited, in excellent agreement with earlier theoretical predictions. However, the distributions over the OD(X2Π) fine-structure and Λ-doublet states, diagnostic of electronic non-adiabaticity in the reaction, challenge the prevailing theoretical understanding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tsang, W. & Hampson, R. F. Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J. Phys. Chem. Ref. Data 15, 1087–1279 (1986).
Balakrishnan, N. Quantum calculations of the O(3P) + H2 → OH + H reaction. J. Chem. Phys. 121, 6346–6352 (2004).
Neumark, D. M., Wodtke, A. M., Robinson, G. N., Hayden, C. C. & Lee, Y. T. Molecular-beam studies of the F + H2 reaction. J. Chem. Phys. 82, 3045–3066 (1985).
Jankunas, J. et al. Seemingly anomalous angular distributions in H + D2 reactive scattering. Science 336, 1687–1690 (2012).
Che, L. et al. Breakdown of the Born–Oppenheimer approximation in the F + o-D2 → DF + D reaction. Science 317, 1061–1064 (2007).
Walch, S. P., Dunning, T. H. Jr, Raffenetti, R. C. & Bobrowicz, F. W. A theoretical study of the potential energy surface for O(3P) + H2 . J. Chem. Phys. 72, 406–415 (1980).
Walch, S. P., Wagner, A. F., Dunning, T. H. Jr & Schatz, G. C . Theoretical studies of the O + H2 reaction. J. Chem. Phys. 72, 2894–2896 (1980).
Bowman, J. M., Wagner, A. F., Walch, S. P. & Dunning, T. H. Jr. Reaction dynamics for O(3P) + H2 and D2. IV. Reduced dimensionality quantum and quasiclassical rate constants with an adiabatic incorporation of the bending motion. J. Chem. Phys. 81, 1739–1752 (1984).
Rogers, S., Wang, D., Kuppermann, A. & Walch, S. Chemically accurate ab initio potential energy surfaces for the lowest 3A′ and 3A′′ electronically adiabatic states of O(3P) + H2 . J. Phys. Chem. A 104, 2308–2325 (2000).
Hoffmann, M. R. & Schatz, G. C. Theoretical studies of intersystem crossing effects in the O + H2 reaction. J. Chem. Phys. 113, 9456–9465 (2000).
Garton, D. J., Minton, T. K., Maiti, B., Troya, D. & Schatz, G. C. A crossed molecular beams study of the O(3P) + H2 reaction: comparison of excitation function with accurate quantum reactive scattering calculations. J. Chem. Phys. 118, 1585–1588 (2003).
Braunstein, M., Adler-Golden, S., Maiti, B. & Schatz, G. C. Quantum and classical studies of the O(3P) + H2 (v = 0–3, j = 0) → OH + H reaction using benchmark potential surfaces. J. Chem. Phys. 120, 4316–4323 (2004).
Garton, D. J. et al. Experimental and theoretical investigations of the inelastic and reactive scattering dynamics of O(3P) + D2 . J. Phys. Chem. A 110, 1327–1341 (2006).
Chu, T-S., Zhang, X. & Han, K-L. A quantum wave-packet study of intersystem crossing effects in the O(3P2,1,0,1D2) + H2 reaction. J. Chem. Phys. 122, 214301 (2005).
Garashchuk, S., Rassolov, V. A. & Schatz, G. C. Semiclassical nonadiabatic dynamics based on quantum trajectories for the O(3P,1D) + H2 system. J. Chem. Phys. 124, 244307 (2006).
Li, B. & Han, K-L. Mixed quantum–classical study of nonadiabatic dynamics in the O(3P2,1,0,1D2) + H2 reaction. J. Phys. Chem. A 113, 10189–10195 (2009).
Xu, Z. & Zong, F. Chemical stereodynamics of the O(3P) + H2 (v = 0, j = 0) → OH + H reaction on the two lowest triplet electronic states. J. Mol. Struct Theochem 960, 22–30 (2010).
Han, B. & Zheng, Y. Nonadiabatic quantum dynamics in O(3P) + H2 → OH + H: a revisited study. J. Comput. Chem. 32, 3520–3525 (2011).
Maiti, B. & Schatz, G. C. Theoretical studies of intersystem crossing effects in the O(3P,1D) + H2 reaction. J. Chem. Phys. 119, 12360–12371 (2003).
Han, J., Chen, X. & Weiner, B. R. Reaction dynamics of O(3P) + H2 (v = 1). Chem. Phys. Lett. 332, 243–250 (2000).
Dieke, G. H. & Crosswhite, H. M. The ultraviolet bands of OH: fundamental data. J. Quant. Spectrosc. Radiat. Transfer 2, 97–199 (1962).
Alexander, M. H. et al. A nomenclature for Λ-doublet levels in rotating linear molecules. J. Chem. Phys. 89, 1749–1753 (1988).
Luque, J & Crosley, D. R. LIFBASE: Database and Spectral Simulation Program (Version 1.5), SRI International Report MP 99-009 (SRI International, Menlo Park, California, 1999).
Andresen, P. & Rothe, E. W. Analysis of chemical dynamics via Λ-doubling: Directed lobes in product molecules and transition states. J. Chem. Phys. 82, 3634–3640 (1985).
Greene, C. H. & Zare, R. N. Determination of product population and alignment using laser-induced fluorescence. J. Chem. Phys. 78, 6741–6753 (1983).
Bronikowski, M. J. & Zare, R. N. Simple model for Λ-doublet propensities in bimolecular reactions. Chem. Phys. Lett. 166, 5–10 (1990).
Alexander, M. H., Rackham, E. J. & Manolopoulos, D. E. Product multiplet branching in the O(1D) + H2 → OH(2Π) + H reaction. J. Chem. Phys. 121, 5221–5235 (2004).
Butler, J. E., Jursich, G. M., Watson, I. A. & Wiesenfeld, J. R. Reaction dynamics of atomic oxygen (1D2) H2, HD, D2: OH, OD(X2Π) product internal energy distributions. J. Chem. Phys. 84, 5365–5377 (1986).
Caledonia, G. E., Krech, R. H. & Green, B. D. A high flux source of energetic oxygen atoms for material degradation studies. AIAA J. 25, 59–63 (1987).
Auerbach, D. J. Velocity measurements by time of flight methods in Atomic and Molecular Beam Methods Vol. 1 (eds Scoles, G., Bassi, D, Buck, U. & Laine, D. C.) 362–379 (Oxford Univ. Press, 1988).
Acknowledgements
This work was supported by the Air Force Office of Scientific Research (FA9550-10-1-0563). K.G.M. is grateful for a Royal Society Leverhulme Trust Senior Research Fellowship under which this work was initiated. We thank G. Schatz, M. Costen and A. Orr-Ewing for invaluable discussions and advice. The data described in this work can be obtained from the corresponding authors on request.
Author information
Authors and Affiliations
Contributions
T.K.M. and J.Z. conceived and designed the experiments. J.Z., S.L. and K.G.M. performed the experiments and analysed the data. S.L., J.Z., K.G.M. and T.K.M. contributed to the analysis methods, discussed the results and commented on the manuscript. K.G.M. and T.K.M. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 658 kb)
Rights and permissions
About this article
Cite this article
Lahankar, S., Zhang, J., McKendrick, K. et al. Product-state-resolved dynamics of the elementary reaction of atomic oxygen with molecular hydrogen, O(3P) + D2 → OD(X2Π) + D. Nature Chem 5, 315–319 (2013). https://doi.org/10.1038/nchem.1588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1588
This article is cited by
-
Product lambda-doublet ratios as an imprint of chemical reaction mechanism
Nature Communications (2016)
-
OH electron, where art thou?
Nature Chemistry (2013)