Abstract
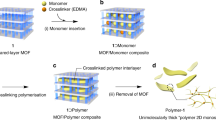
Chain alignment can significantly influence the macroscopic properties of a polymeric material, but no general and versatile methodology has yet been reported to obtain highly ordered crystalline packing of polymer chains, with high stability. Here, we disclose a strategy that relies on ‘ordered crosslinks’ to produce polymeric materials that exhibit a crystalline arrangement. Divinyl crosslinkers (2,5-divinyl-terephthalate) were first embedded, as substitutional ligands, into the structure of a porous coordination polymer (PCP), [Cu(terephthalate)triethylenediamine0.5]n. A representative vinyl monomer, styrene, was subsequently polymerized inside the channels of the host PCP. The polystyrene chains that form within the PCP channels also crosslink with the divinyl species. This bridges together the polymer chains of adjacent channels and ensures that, on selective removal of the PCP, the polymer chains remain aligned. Indeed, the resulting material exhibits long-range order and is stable to thermal and solvent treatments, as demonstrated by X-ray powder diffraction and transmission electron microscopy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martin, C. R. Template synthesis of electronically conductive polymer nanostructures. Acc. Chem. Res. 28, 61–68 (1995).
Akagi, K. & Shirakawa, H. Morphological alignment of liquid crystalline conductive polyacetylene derivatives. Macromol. Symp. 104, 137–158 (1996).
Lee, J. I. et al. Highly aligned ultrahigh density arrays of conducting polymer nanorods using block copolymer templates. Nano Lett. 8, 2315–2320 (2008).
Natta, G., Corradini, P. & Bassi, I. B. Crystal structure of isotactic polystyrene. Il Nuovo Cimento 15, 68–82 (1960).
Farina, M. The stereochemistry of linear macromolecules. Top. Stereochem. 17, 1–111 (1987).
Kaminsky, W. Highly active metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1413–1418 (1998).
Toney, M. F. et al. Near-surface aligment of polymers in rubbed films. Nature 347, 709–711 (1995).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Kubo, Y. et al. A supramolecular bundling approach toward the alignment of conjugated polymers. Angew. Chem. Int. Ed. 45, 1548–1553 (2006).
Sozzani, P., Bovey, F. A. & Schilling, F. C. Characterization of isolated polyethylene chains in the solid state. Macromolecules 24, 6764–6768 (1991).
Lauher, J. W., Fowler, F. W. & Goroff, N. S. Single-crystal-to-single-crystal topochemical polymerizations by design. Acc. Chem. Res. 41, 1215–1229 (2008).
Matsumoto, A. Polymer structure control based on crystal engineering for material design. Polym. J. 35, 93–121 (2003).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nature Chem. 4, 287–291 (2012).
Grell, M., Bradley, D. D. C., Inbasekaran, M. & Woo, E. P. A glass-forming conjugated main-chain liquid crystal polymer for polarized electroluminescence applications. Adv. Mater. 9, 798–802 (1997).
Schmidt, B. V. K. J., Fechler, N., Falkenhagen, J. & Lutz, J-F. Controlled folding of synthetic polymer chains through the formation of positionable covalent bridges. Nature Chem. 3, 234–238 (2011).
Svec, F. & Fréchet, J. M. J. New design of macroporous polymers and supports: from separation to biocatalysis. Science 273, 205–211 (1996).
Malik, M. A., Ali, S. W. & Ahmed, I. Sulfonated styrene-divinylbenzene resins: optimizing synthesis and estimating characteristics of the base copolymers and the resins. Ind. Eng. Chem. Res. 49, 2608–2612 (2010).
O'Keeffe, M. & Yaghi, O. M. Deconstructing the crystal structures of metal–organic frameworks and related materials into their underlying nets. Chem. Rev. 112, 675–702 (2012).
Ferey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Murray, L. J., Dinca, M. & Long, J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Li, J-R., Sculley, J. & Zhou, H. C. Metal–organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
Shimizu, G. K. H., Vaidhyanathan, R. & Taylor J. M. Phosphonate and sulfonate metal organic frameworks. Chem. Soc. Rev. 38, 1430–1449 (2009).
Cohen, S. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 112, 970–1000 (2012).
Wang, Z., Chen, G. & Ding, K. Self-suported catalysts. Chem. Rev. 109, 322–359 (2009).
Corma, A., Garcia, H. & Llabrés i Xamena, F. X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 110, 4606–4655 (2010).
Uemura, T., Yanai, N. & Kitagawa, S. Polymerization reactions in porous coordination polymers. Chem. Soc. Rev. 38, 1228–1236 (2009).
Lee, J. Y. et al. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 (2009).
Uemura, T., Ono, Y., Kitagawa, K. & Kitagawa, S. Radical polymerization of vinyl monomers in porous coordination polymers: nanochannel size effects on reactivity, molecular weight and stereostructure. Macromolecules 41, 87–94 (2008).
Uemura, T., Ono, Y., Hijikata, Y. & Kitagawa, S. Functionalization of coordination nanochannels for controlling tacticity in radical vinyl polymerization. J. Am. Chem. Soc. 132, 4917–4924 (2010).
Uemura, T. et al. Conformation and molecular dynamics of single polystyrene chain confined in coordination nanospace. J. Am. Chem. Soc. 130, 6781–6788 (2008).
Fukushima, T. et al. Solid solution of soft porous coordination polymers: fine-tuning of gas adsorption properties. Angew. Chem. Int. Ed. 49, 4820–4824 (2010).
Deng, H. et al. Multiple functional groups of varying ratios in metal–organic frameworks. Science 327, 846–850 (2010).
Carson, C. G. et al. Synthesis and structure characterization of copper terephthalate metal–organic frameworks. Eur. J. Inorg. Chem. 16, 2338–2343 (2009).
Vinagradov, E., Madhu, P. K. & Vega, S. High-resolution proton solid-state NMR spectroscopy by phase-modulated Lee–Goldburg experiment. Chem. Phys. Lett. 314, 443–450 (1999).
Glans, J. H. & Turner, D. T. Glass transition elevation of polystyrene by crosslinks. Polymer 22, 1540–1543 (1981).
Li, Y., Fan, Y. & Ma, J. Thermal, physical and chemical stability of porous polystyrene-type beads with different degrees of crosslinking. Polym. Degrad. Stab. 73, 163–167 (2001).
Sozzani, P. et al. Complete shape retention in the transformation of silica to polymer micro-objects. Nature Mater. 5, 545–551 (2006).
Distefano, G., Comotti, A., Bracco, S., Beretta, M. & Sozzani, P. Porous dipeptide crystals as polymerization nanoreactors. Angew. Chem. Int. Ed. 51, 9258–9262 (2012).
Cepak, V. M. & Martin, C. R. Preparation of polymeric micro- and nanostructures using a template-based deposition method. Chem. Mater. 11, 1363–1367 (1999).
Moon, S. I. & McCarthy, T. J. Template synthesis and self-assembly of nanoscopic polymer ‘pencils’. Macromolecules 36, 4253–4255 (2003).
Thomas, A., Goettmann, F. & Antonietti, M. Hard templates for soft materials: creating nanostructured organic materials. Chem. Mater. 20, 738–755 (2008).
Johnson, S. A., Brigham, E. S., Ollivier, P. J. & Mallouk, T. E. Effect of micropore topology on the structure and properties of zeolite polymer replicas. Chem. Mater. 9, 2448–2458 (1997).
Nyquist, R. A., Putzig, C. L., Leugers, M. A., McLachlan, R. D. & Thill, B. Comparison of the vibrational spectra and assignments for α-syndiotactic, β-syndiotactic, isotactic and atactic polystyrene and toluene. Appl. Spectrosc. 46, 981–987 (1992).
Reynolds, N. M., Savage, J. D. & Hsu, S. L. A spectroscopic study of syndiotactic polystyrene. Macromolecules 22, 2867–2869 (1989).
Kobayashi, M., Nakaoki, T. & Ishihara, N. Molecular conformation in glasses and gels of syndiotactic and isotactic polystyrenes. Macromolecules 23, 78–83 (1990).
Krimm, S. & Tobolsky, A. V. Quantitative X-ray studies of order in amorphous and crystalline polymers: scattering from various polymers and a study of the glass transition in polystyrene and polymethyl methacrylate. Text. Res. J. 21, 805–822 (1951).
White, D. M. Stereospecific polymerization in urea canal complexes. J. Am. Chem. Soc. 82, 5678–5685 (1960).
Di Silvestro, G. & Sozzani, P. Polymerization in clathrates. Comprehensive Polymer Science 4, 303–315 (1989).
Allcock, H. R., Silverberger, E. N. & Dudley, G. K. Stereocontrolled polymerization within a cyclophosphazene clathrate tunnel system. Macromolecules 27, 1033–1038 (1994).
Ishihara, N., Seimiya, T., Kuramoto, M. & Uoi, M. Crystalline syndiotactic polystyrene. Macromolecules 19, 2464–2465 (1986).
Wang, L-H., Choy, C. L. & Porter, R. S. Thermal expansion of ultradrawn polystyrene. J. Polym. Sci. 20, 633–640 (1982).
Brandrup, J. & Immergut, E. H. Polymer Handbook 3rd edn (Wiley-Interscience, 1989).
Boyer, R. F. in Encyclopedia of Polymer Science and Technology Vol. 13 (ed. Mark, H. F.) 251–326 (Wiley, 1970).
Hay, J. N. Crystallization kinetics of high polymers: isotactic polystyrene. J. Polym. Sci. A 3, 433–447 (1965).
Levchik, G. F., Si, K., Levchik, S. V., Camino, G. & Wilkie, C. A. The correlation between cross-linking and thermal stability: cross-linked polystyrenes and polymethacrylates. Polym. Degrad. Stab. 65, 395–403 (1999).
Bonifacio, M. C., Robertson, C. R., Jung, J. Y. & King, B. T. Polycyclic aromatic hydrocarbons by ring-closing metathesis. J. Org. Chem. 70, 8522–8526 (2005).
Acknowledgements
This work was supported by the Asahi Glass Foundation and a Grant-in-Aid for Young Scientists (A) from the Ministry of Education, Culture, Sports, Science and Technology, Government of Japan. A.C. and P.S. thank the Cariplo Foundation and Lombardy Region for financial support. G.D. and T.U. thank Y. Chujo of Kyoto University for access to SEM-EDX apparatus. S.I. thanks H. Kurata of Kyoto University for use of the cryogenic TEM.
Author information
Authors and Affiliations
Contributions
T.U. conceived and directed the project. G.D. designed and performed the experiments. H.S. carried out the experiments. M.T. and S.I. performed TEM measurements and analysed the data. S.B., A.C. and P.S. performed the solid-state NMR measurements. G.D., A.C., P.S., T.U. and S.K. discussed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8110 kb)
Rights and permissions
About this article
Cite this article
Distefano, G., Suzuki, H., Tsujimoto, M. et al. Highly ordered alignment of a vinyl polymer by host–guest cross-polymerization. Nature Chem 5, 335–341 (2013). https://doi.org/10.1038/nchem.1576
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1576
This article is cited by
-
Controlled assemblies of conjugated polymers in metal−organic frameworks
Polymer Journal (2022)
-
Confined polymerization: RATRP of GMA in the bicontinuous PolyHIPE
Journal of Polymer Research (2022)
-
Complex polymer architectures through free-radical polymerization of multivinyl monomers
Nature Reviews Chemistry (2020)
-
Unimolecularly thick monosheets of vinyl polymers fabricated in metal–organic frameworks
Nature Communications (2020)
-
Computer-aided discovery of connected metal-organic frameworks
Nature Communications (2019)