Abstract
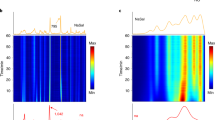
Chemical and structural transformations have long been carried out by milling. Such mechanochemical steps are now ubiquitous in a number of industries (such as the pharmaceutical, chemical and metallurgical industries), and are emerging as excellent environmentally friendly alternatives to solution-based syntheses. However, mechanochemical transformations are typically difficult to monitor in real time, which leaves a large gap in the mechanistic understanding required for their development. We now report the real-time study of mechanochemical transformations in a ball mill by means of in situ diffraction of high-energy synchrotron X-rays. Focusing on the mechanosynthesis of metal–organic frameworks, we have directly monitored reaction profiles, the formation of intermediates, and interconversions of framework topologies. Our results reveal that mechanochemistry is highly dynamic, with reaction rates comparable to or greater than those in solution. The technique also enabled us to probe directly how catalytic additives recently introduced in the mechanosynthesis of metal–organic frameworks, such as organic liquids or ionic species, change the reactivity pathways and kinetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
08 January 2013
In the version of this Article originally published, EPSRC and J. K. M. Sanders should have been included in the Acknowledgements section; this has now been corrected in the HTML and PDF versions of this Article.
References
Takacs, L. Quicksilver from cinnabar: the first documented mechanochemical reaction? J. Minerals Metals Mater. Soc. 52, 12–13 (2000).
James, S. L. et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 41, 413–447 (2012).
Baláž, P. & Dutková, E. Fine milling in applied mechanochemistry. Miner. Eng. 22, 681–694 (2009).
Janot, R. & Guérard, D. Ball-milling in liquid media: applications to the preparation of anodic materials for lithium-ion batteries. Prog. Mater. Sci. 50, 1–92 (2005).
Bruckmann, A., Krebs, A. & Bolm, C. Organocatalytic reactions: effects of ball milling, microwave and ultrasound irradiation. Green Chem. 10, 1131–1141 (2008).
Stolle, A., Szuppa, T., Leonhardt, S. E. S. & Ondruschka, B. Ball milling in organic synthesis: solutions and challenges. Chem. Soc. Rev. 40, 2317–2329 (2011).
Lazuen-Garay, A., Pichon, A. & James, S. L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 36, 846–855 (2007).
Adams, C. J., Haddow, M. F., Lusi, M. & Orpen, A. G. Crystal engineering of lattice metrics of perhalometallate salts and MOFs. Proc. Natl Acad. Sci. USA 107, 16033–16038 (2010).
Beldon, P. J. et al. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. Int. Ed. 49, 9640–9643 (2010).
André, V. M. et al. Mechanosynthesis of the metallodrug bismuth subsalicylate from Bi2O3 and structure of bismuth salicylate without auxiliary organic ligands. Angew. Chem. Int. Ed. 50, 7858–7861 (2011).
Baláž, P. & Dutková, E. Mechanochemistry of sulphides, from minerals to advanced nanocrystalline materials. J. Therm. Anal. Cal. 90, 85–92 (2007).
Rodríguez, B., Bruckmann, A., Rantanen, T. & Bolm, C. Solvent-free carbon–carbon bond formations in ball mills. Adv. Synth. Catal. 349, 2213–2233 (2007).
Delori, A., Friščić, T. & Jones, W. The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 14, 2350–2362 (2012).
Daurio, D., Medina, C., Saw, R., Nagapudi, K. & Alvarez-Núñez, F. Application of twin screw extrusion in the manufacture of cocrystals, part I: four case studies. Pharmaceutics 3, 582–600 (2011).
Nguyen, K. L., Friščić, T., Day, G. M., Gladden, L. F. & Jones, W. Nature Mater. 6, 206–209 (2007).
Friščić, T. et al. Ion- and liquid-assisted grinding: improved mechanochemical synthesis of metal–organic frameworks reveals salt inclusion and anion templating. Angew. Chem. Int. Ed. 49, 712–715 (2010).
Friščić, T. & Jones, W. Recent advances in understanding the mechanism of cocrystal formation via grinding. Cryst. Growth Des. 9, 1621–1637 (2009).
Urakaev, F. Kh. & Boldyrev, V. V. Mechanism and kinetics of mechanochemical processes in comminuting devices 1. Theory. Powder Technol. 107, 93–107 (2000).
Gutman, E. M. Mechanochemistry of Materials (Cambridge International Science, 1998).
Kaupp, G. Solid-state molecular syntheses: complete reactions without auxiliaries based on the new solid-state mechanism. CrystEngComm 5, 117–133 (2003).
Rastogi, R. P. & Singh, N. B. Solid-state reactivity of picric acid and substituted hydrocarbons. J. Phys. Chem. 72, 4446–4449 (1968).
Rothenberg, G., Downie, A. P., Raston, C. L. & Scott, J. L. Understanding solid/solid organic reactions. J. Am. Chem. Soc. 123, 8701–8708 (2001).
Tumanov, I. A., Achkasov, A. F., Boldyreva, E. V. & Boldyrev, V. V. Following the products of mechanochemical synthesis step by step. CrystEngComm 13, 2213 (2011).
Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 47, 355–414 (2002).
Cinčić, D., Friščić, T. & Jones, W. Stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 130, 7524–7525 (2008).
Štrukil, V. et al. Towards an environmentally-friendly laboratory: dimensionality and reactivity in the mechanosynthesis of metal–organic compounds. Chem. Commun. 46, 9191–9193 (2010).
Braga, D. et al. Mechanochemical preparation of molecular and supramolecular organometallic materials and coordination networks. J. Chem. Soc. Dalton Trans. 1249–1263 (2006).
Braga, D., Grepioni, F. & Lampronti, G. I. Supramolecular metathesis: co-former exchange in co-crystals of pyrazine with (R,R)-, (S,S)-, (R,S)- and (S,S/R,R)-tartaric acid. CrystEngComm 13, 3122–3124 (2011).
Bowmaker, G. A. et al. Solution and mechanochemical syntheses, and spectroscopic and structural studies in the silver(I) (bi-)carbonate: triphenylphosphine system. J. Chem. Soc. Dalton Trans. 40, 7210–7218 (2011).
Ibrahim, A. Y., Forbes, R. T. & Blagden, N. Spontaneous crystal growth of co-crystals: the contribution of particle size reduction and convection mixing of the co-formers. CrystEngComm 13, 1141–1152 (2011).
Fujii, K. et al. Direct structure elucidation by powder X-ray diffraction of a metal–organic framework material prepared by solvent-free grinding. Chem. Commun. 46, 7572–7574 (2010).
Adams, C. J., Haddow, M. F. & Orpen, A. G. Crystal synthesis of 1,4-phenylenediamine salts and coordination networks. CrystEngComm 13, 4324–4331 (2011).
Zhang, J-P., Zhang, Y-B., Lin, J-B. & Chen, X-M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 112, 1001–1033 (2012).
Friščić, T., Childs, S. L., Rizvi, S. A. A. & Jones, W. Qualitative view of the role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 11, 418–426 (2009).
Pawley, G. S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 14, 357–361 (1981).
Venna, S. R., Jasinski, J. B. & Carreon, M. A. Structural evolution of zeolitic imidazolate framework–8. J. Am. Chem. Soc. 132, 18030–18033 (2010).
Cravillon, J. et al. Fast nucleation and growth of ZIF-8 nanocrystals monitored by time-resolved in situ small-angle and wide-angle X-ray scattering. Angew. Chem. Int. Ed. 50, 8067–8081 (2011).
Huang, X-C., Lin, Y-Y., Zhang, J-P. & Chen, X-M. Ligand-directed strategy for zeolite-type metal–organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 45, 1557–1559 (2006).
Fernández-Bertrán, J., Castellanos-Serra, L., Yee-Madeira, H. & Reguera, E. Proton transfer in solid state: mechanochemical reactions of imidazole with metallic oxides. J. Solid State Chem. 147, 561–564 (1999).
Spencer, E. C., Angel, R. J., Ross, N. L., Hanson, B. E. & Howard, J. A. K. Pressure-induced cooperative bond rearrangement in a zinc imidazolate framework: a high-pressure single-crystal X-ray diffraction study. J. Am. Chem. Soc. 131, 4022–4026 (2009).
Park, K. S. et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA 103, 10186–10191 (2006).
Martins, G. A. V. et al. The use of ionic liquids in the synthesis of zinc imidazolate frameworks. J. Chem. Soc. Dalton Trans. 39, 1758–1762 (2010).
Bowmaker, G. A., Hanna, J. V., Skelton, B. W. & White, A. H. Solvent-assisted solid-state synthesis: separating the chemical from the mechanical in mechanochemical synthesis. Chem. Commun. 2168–2170 (2009).
Cumbrera, F. L. & Sánchez-Bajo, F. The use of JMAYK kinetic equation for the analysis of solid-state reactions: critical considerations and recent interpretations. Thermochim. Acta 266, 315–330 (1995).
Khawam, A. & Flanagan, D. R. Solid-state kinetic models: basics and mathematical fundamentals. J. Phys. Chem. B 110, 17315–17328 (2006).
Williams, G. R. & O'Hare D. O. J. Phys. Chem. B 110, 10619–10629 (2006).
Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering (Springer-Verlag, 2010).
Wilmer, C. E. et al. Large-scale screening of hypothetical metal–organic frameworks. Nature Chem. 4, 83–89 (2012).
Sonneveld, E. J. & Visser, J. W. Automatic collection of powder data from photographs. J. Appl. Crystallogr. 8, 1–7 (1975).
Hinrichsen, B. Dinnebier, R. E. & Jansen, M. Powder3D: an easy to use program for data reduction and graphical presentation of large numbers of powder diffraction patterns. Z. Kristallogr. 23 (Suppl), 231–236 (2006).
Rietveld, H. M. A profile refinement for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969).
Acknowledgements
The authors acknowledge financial support from the Herchel Smith Fund, the British Council/DAAD (grant no. 1377), ESRF Grenoble, NanoDTC, the University of Cambridge, the Ministry of Science, Education and Sports of the Republic of Croatia and EPSRC, as well as a research fellowship (T.F.) and a doctoral fellowship (P.J.B.). McGill University and FQRNT Centre for Green Chemistry and Catalysis are acknowledged for support. The authors thank A.K. Cheetham for comments, W. Jones for support in acquiring the instrumentation and R.C. Nightingale for equipment design and manufacture and J. K. M. Sanders for support. The assistance of A. Kovač and V. Dunjko with graphics preparation is acknowledged.
Author information
Authors and Affiliations
Contributions
The research was organized by T.F., I.H. and R.E.D. Experiments were performed by T.F., I.H., P.J.B., A.M.B., F.A., S.A.J.K. and V.H. Data analysis was performed by I.H., S.A.J.K., T.F., P.J.B. and R.E.D. The manuscript was written by T.F. and I.H., and graphical materials were prepared by I.H., T.F. and P.J.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4570 kb)
Supplementary Movie 1
Supplementary Movie 1 (AVI 19406 kb)
Rights and permissions
About this article
Cite this article
Friščić, T., Halasz, I., Beldon, P. et al. Real-time and in situ monitoring of mechanochemical milling reactions. Nature Chem 5, 66–73 (2013). https://doi.org/10.1038/nchem.1505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1505
This article is cited by
-
Leveraging mechanochemistry for sustainable polymer degradation
Polymer Journal (2024)
-
Enhancing catalytic activity of UiO-66 through CuO nanoparticles incorporation: a study on Henry reaction and one-pot allylic C-H bond oxidation of olefins
Journal of Chemical Sciences (2024)
-
Mechanochemical synthesis of halogenated heterocyclic compounds
Chemistry of Heterocyclic Compounds (2023)
-
Chemical effects induced by the mechanical processing of granite powder
Scientific Reports (2022)
-
Advancing mechanochemical synthesis by combining milling with different energy sources
Nature Reviews Chemistry (2022)