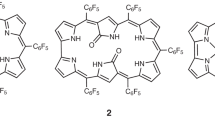
Abstract
Proton-coupled electron transfer (PCET) processes are among the most important phenomena that control a variety of chemical and biological transformations. Although extensively studied in a variety of natural systems and discrete metal complexes, PCET mechanisms are less well codified in the case of purely organic compounds. Here we report that a planar β,β′-phenylene-bridged hexaphyrin (1.0.1.0.1.0), a 24 π-electron antiaromatic species termed rosarin, displays unique redox reactivity on protonation. Specifically, treatment with acid (for example, HI) yields a 26 π-electron aromatic triprotonated monocationic species that is produced spontaneously via an intermediate—but stable—25 π-electron non-aromatic triprotonated monoradical dication. This latter species is also produced on treatment of the original 24 π-electron antiaromatic starting material with HCl or HBr. The stepwise nature of the proton-coupled reduction observed in the planar rosarin stands in marked contrast to that seen for non-annulated rosarins and other ostensibly antiaromatic expanded porphyrinoids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huynh, M. H. V. & Meyer, T. J. Proton-coupled electron transfer. Chem. Rev. 107, 5004–5064 (2007).
Fukuzumi, S. & Kotani, H. in Proton-Coupled Electron Transfer, a Carrefour of Chemical Reactivity Traditions (eds Formosinho, S. & Barroso, M.) 89–125 (Royal Society of Chemistry, 2012).
Costentin, C., Robert, M. & Savéant, J-M. Concerted proton–electron transfers: electrochemical and related approaches. Acc. Chem. Res. 43, 1019–1029 (2010).
Weigberg, D. R. et. al. Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093 (2012).
Gagliardi, C. J. et al. Integrating proton coupled electron transfer (PCET) and excited states. Coord. Chem. Rev. 254, 2459–2471 (2010).
Meyer, T. J., Huynh, M. H. V. & Thorp, H. H. The possible role of proton-coupled electron transfer (PCET) in water oxidation by photosystem II. Angew. Chem. Int. Ed. 46, 5284–5304 (2007).
McEvoy, J. P. & Brudvig, G. W. Water-splitting chemistry of photosystem II. Chem. Rev. 106, 4455–4483 (2006).
Kaila, V. R. I., Verkhovsky, M. I. & Wikström, M. Proton-coupled electron transfer in cytochrome oxidase. Chem. Rev. 110, 7062–7081 (2010).
Litwinienko, G. & Ingold, K. U. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 40, 222–230 (2007).
Warren, J. J. & Mayer, J. M. Surprisingly long-lived ascorbyl radicals in acetonitrile: concerted proton–electron transfer reactions and thermochemistry. J. Am. Chem. Soc. 130, 7546–7547 (2008).
Sessler, J. L. & Seidel, D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Ed. 42, 5134–5175 (2003).
Saito, S. & Osuka, A. Expanded porphyrins: intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 50, 4342–4373 (2011).
Osuka, A. & Saito, S. Expanded porphyrins and aromaticity. Chem. Commun. 47, 4330–4339 (2011).
Sessler, J. L. et al. Rosarin: a new, easily prepared hexapyrrolic expanded porphyrin. J. Am. Chem. Soc. 114, 8306–8307 (1992).
Lament, B., Dobkowski, J., Sessler, J. L., Weghorn, S. J. & Waluk, J. Spectroscopy and photophysics of a highly nonplanar expanded porphyrin: 4,9,13,18,22,27-hexaethyl-5,8,14,17,23,26-hexamethyl-2,11,20-triphenylrosarin. Chem. Eur. J. 5, 3039–3045 (1999).
Setsune, J., Katakami, Y. & Iizuna, N. [48]Dodecaphyrin-(1.0.1.0.1.0.1.0.1.0.1.0) and [64]hexadecaphyrin-(1.0.1.0.1.0.1.0.1.0.1.0.1.0.1.0): the largest cyclopolypyrroles. J. Am. Chem. Soc. 121, 8957–8958 (1999).
Garratt, P. J. Aromaticity (Wiley, 1986).
Cho, S. et al. Defining spectroscopic features of heteroannulenic antiaromatic porphyrinoids. J. Phys. Chem. Lett. 1, 895–900 (2010).
Steiner, E. & Fowler, P. W. The shell structure of π ring currents in the expanded porphyrin amethyrin. Org. Biomol. Chem. 4, 2473–2476 (2006).
Wallenborn, E-U., Haldimann, R. F., Klärner, F-G. & Diederich, F. Theoretical investigation of the origin of regioselectivity in the formation of methanofullerenes by addition of diazo compounds: a model study. Chem. Eur. J. 4, 2258–2265 (1998).
Yoon, M-C., Cho, S., Suzuki, M., Osuka, A. & Kim, D. Aromatic versus antiaromatic effect on photophysical properties of conformationally locked trans-vinylene-bridged hexaphyrins. J. Am. Chem. Soc. 131, 7360–7367 (2009).
Yoon, Z. S. et al. Nonlinear optical properties and excited-state dynamics of highly symmetric expanded porphyrins. J. Am. Chem. Soc. 128, 14128–14134 (2006).
Mori, S. et al. Peripheral fabrications of a bis-gold(III) complex of [26]hexaphyrin(1.1.1.1.1.1) and aromatic versus antiaromatic effect on two-photon absorption cross section. J. Am. Chem. Soc. 129, 11344–11345 (2007).
Lim, J. M. et al. The photophysical properties of expanded porphyrins: relationships between aromaticity, molecular geometry and non-linear optical properties. Chem. Commun. 261–273 (2009).
Yoon, Z. S., Osuka, A. & Kim, D. Möbius aromaticity and antiaromaticity in expanded porphyrins. Nature Chem. 1, 113–122 (2009).
Choi, J-H., Ahn, I-H., Sessler, J. L. & Cho, D-C. Colorimetric iodide detection in water: a new photo-activated indicator system. Supramolecular Chem. 23, 283–286 (2011).
Fukuzumi, S., Mochizuki, S. & Tanaka, T. Efficient catalytic systems for electron transfer from an NADH model compound to dioxygen. Inorg. Chem. 29, 653–659 (1990).
Boschloo, G. & Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 42, 1819–1826 (2009).
Connelly, N. G. & Geiger, W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Seidel, D., Lynch, V. & Sessler, J. L. Cyclo[8]pyrrole: a simple-to-make expanded porphyrin with no meso bridges. Angew. Chem. Int. Ed. 41, 1422–1425 (2002).
Köhler, T. et. al. Formation and properties of cyclo[6]pyrrole and cyclo[7]pyrrole. J. Am. Chem. Soc. 125, 6872–6873 (2003).
Shin, J-Y. et al. Aromaticity and photophysical properties of various topology-controlled expanded porphyrins. Chem. Soc. Rev. 39, 2751–2767 (2010).
Pawlicki, M., Collins, H. A., Denning, R. G. & Anderson, H. L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. 48, 3244–3266 (2009).
Roznyatovskiy, V., Lynch, V. & Sessler, J. L. Dinaphthoporphycenes. Org. Lett. 12, 4424–4427 (2010).
Acknowledgements
Support is acknowledged from the US National Science Foundation (CHE-1057904 to J.L.S.), the Robert A. Welch Foundation (F-1018 to J.L.S.), Grant-in-Aid (No. 20108010 to S.F. and 23750014 to K.O.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the World Class University Program (R32-2010-000-10217-0 to D.K. and R31-2008-000-10010-0 to S.F.) of the Ministry of Education, Science and Technology, the Basic Science Research Programs, National Research Foundation (2009-0087013 to C-H.L), BK21, Council of Scientific and Industrial Research, India (from a senior research fellowship to T.S.) and the Department of Science and Technology India (SR/S1/IC-20/2007 to P.K.P.).
Author information
Authors and Affiliations
Contributions
J.L.S. supervised the project. M.I. designed and carried out the bulk of the experimental and theoretical work. S-J.K., C.P., V.V.R., T.S. and P.K.P. performed the syntheses. K.O. carried out the EPR experiment. J.M.L. carried out TA experiments. B.S.L. carried out two photon-absorption experiments. C.P., V.M.L. and J.S.P. carried out X-ray diffraction analyses on the crystals. M.I., C-H.L., S.F., D.K. and J.L.S. co-wrote the paper. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6972 kb)
Supplementary information
Crystallographic data for compound 1. (CIF 38 kb)
Supplementary information
Crystallographic data for compound H31·Cl. (CIF 38 kb)
Rights and permissions
About this article
Cite this article
Ishida, M., Kim, SJ., Preihs, C. et al. Protonation-coupled redox reactions in planar antiaromatic meso-pentafluorophenyl-substituted o-phenylene-bridged annulated rosarins. Nature Chem 5, 15–20 (2013). https://doi.org/10.1038/nchem.1501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1501
This article is cited by
-
Exploration of pyrazine-embedded antiaromatic polycyclic hydrocarbons generated by solution and on-surface azomethine ylide homocoupling
Nature Communications (2017)
-
Bicyclic Baird-type aromaticity
Nature Chemistry (2017)
-
Two-electron Oxidation of a Twisted Non Anti-aromatic 40π Expanded Isophlorin
Journal of Chemical Sciences (2016)