Abstract
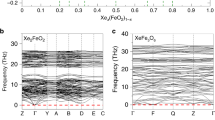
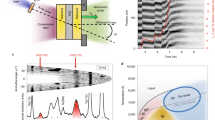
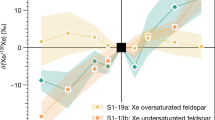
Xenon, which is quite inert under ambient conditions, may become reactive under pressure. The possibility of the formation of stable xenon oxides and silicates in the interior of the Earth could explain the atmospheric missing xenon paradox. Using an ab initio evolutionary algorithm, we predict the existence of thermodynamically stable Xe–O compounds at high pressures (XeO, XeO2 and XeO3 become stable at pressures above 83, 102 and 114 GPa, respectively). Our calculations indicate large charge transfer in these oxides, suggesting that large electronegativity difference and high pressure are the key factors favouring the formation of xenon compounds. However, xenon compounds cannot exist in the Earth's mantle: xenon oxides are unstable in equilibrium with the metallic iron occurring in the lower mantle, and xenon silicates are predicted to decompose spontaneously at all mantle pressures (<136 GPa). However, it is possible that xenon atoms may be retained at defects in mantle silicates and oxides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levy, H. A. & Agron, P. A. The crystal and molecular structure of xenon difluoride by neutron diffraction. J. Am. Chem. Soc. 85, 241–242 (1963).
Templeton, D. H., Zalkin, A., Forrester, J. D. & Williamson, S. M. Crystal and molecular structure of xenon trioxide. J. Am. Chem. Soc. 85, 817 (1963).
Hoyer, S., Emmler, T. & Seppelt, K. The structure of xenon hexafluoride in the solid state. J. Fluor. Chem. 127, 1415–1422 (2006).
Kim, M., Debessai, M. & Yoo, C. S. Two- and three-dimensional extended solids and metallization of compressed XeF2 . Nature Chem. 2, 784–788 (2010).
Somayazulu, M. et al. Pressure-induced bonding and compound formation in xenon hydrogen solids. Nature Chem. 2, 50–53 (2010).
Smith, D. F. Xenon trioxide. J. Am. Chem. Soc. 85, 816–817 (1963).
Selig, H., Claassen, H. H., Chernick C. L., Malm, J. G. & Huston, L. L. Xenon tetroxide: preparation and some properties. Science 143, 1322–1323 (1964).
Brock, D. S. & Schrobilgen, G. J. Synthesis of the missing oxide of xenon, XeO2, and its implications for Earth's missing xenon. J. Am. Chem. Soc. 133, 6265–6269 (2011).
Grochala, W. Atypical compounds of gases, which have been called ‘noble’. Chem. Soc. Rev. 36, 1632–1655 (2007).
Anders, E. & Owen, T. Mars and Earth: origin and abundance of volatiles. Science 198, 453–465 (1977).
Sanloup, C., Hemley, R. J. & Mao, H. K. Evidence for xenon silicates at high pressure and temperature. Geophys. Res. Lett. 29, 1883–1886 (2002).
Sanloup, C. et al. Retention of xenon in quartz and Earth's missing xenon. Science 310, 1174–1177 (2005).
Oganov, A. R., Ma, Y., Lyakhov, A. O., Valle, M. & Gatti, C. Evolutionary crystal structure prediction as a method for the discovery of minerals and materials. Rev. Mineral. Geochem. 71, 271–298 (2010).
Caldwell, W. A. et al. Structure, bonding, and geochemistry of xenon at high pressures. Science 277, 930–933 (1997).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys. 124, 244704 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works—and why. Acc. Chem. Res. 44, 227–237 (2011).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990).
Oganov, A. R. et al. Ionic high-pressure form of elemental boron. Nature 457, 863–867 (2009).
Bader, R. F. W. Atoms in Molecules—A Quantum Theory (Oxford Univ. Press, 1990).
Frost, J. C. et al. Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409–412 (2004).
Zhang, F. & Oganov, A. R. Valence state and spin transitions of iron in Earth's mantle silicates. Earth Planet. Sci. Lett. 249, 436–443 (2006).
Oganov, A. R., Martonak, R., Laio, A., Raiteri, R. & Parrinello, M. Anisotropy of Earth's D′′ layer and stacking faults in the MgSiO3 post-perovskite phase. Nature 438, 1142–1144 (2005).
Urusov, V. S. & Dudnikova, V. B. The trace-component trapping effect: experimental evidence, theoretical interpretation, and geochemical applications. Geochim. Cosmochim. Acta 62, 1233–1240 (1998).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 3865–3868 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Klimes, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Dovesi, R. et al. CRYSTAL06 User's Manual (University of Torino, 2006).
Towler, M. D. et al. Ab initio study of MnO and NiO. Phys. Rev. B 50, 5041–5054 (1994).
Peterson, K. A. et al. Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16–18 elements. J. Chem. Phys. 119, 11113–11123 (2003).
Gatti, C. TOPOND-98: An Electron Density Topological Program for Systems Periodic in N (N = 0–3) Dimensions (CNR-ISTM, 1999).
Gatti, C. in The Quantum Theory of Atoms in Molecules (eds Matta, C.F. & Boyd, R.J.) 165–206 (Wiley-VCH, 2007).
Keith, T. Molecules in Magnetic Fields. PhD thesis, McMaster Univ. (1993).
Shishkin, M. & Kresse, G. Self-consistent GW calculations for semiconductors and insulators. Phys. Rev. B 75, 235102 (2007).
Ma, Y., Oganov, A. R. & Glass, C. W. Structure of the metallic ζ-phase of oxygen and isosymmetric nature of ζ–ε phase transition: ab initio simulations. Phys. Rev. B 76, 064101 (2007).
Lundegaard, L. F., Weck, G., McMahon, M. I., Desgreniers, S. & Loubeyre, P. Observation of an O8 molecular lattice in the ɛ phase of solid oxygen. Nature 443, 201–204 (2006).
Sears, D. R. & Klug, H. P. Density and expansivity of solid xenon. J. Chem. Phys. 37, 3002–3006 (1962).
Sonnenblick, Y., Alexander, E., Kalman, Z. & Steinberger, I. Hexagonal close packed krypton and xenon. Chem. Phys. Lett. 52, 276–278 (1977).
Boehler, R., Ross, M. & Boercker, D. B. High-pressure melting curves of alkali halides. Phys. Rev. B 53, 556–563 (1996).
Acknowledgements
Calculations were performed on the CFN cluster and Blue Gene supercomputer (Brookhaven National Laboratory), Swiss Supercomputer Centre, Skif MSU supercomputer (Moscow State University) and at the Joint Supercomputer Center of the Russian Academy of Sciences (Moscow). A.R.O. thanks DARPA (grant no. W31P4Q1210008) and the National Science Foundation (grant no. EAR-1114313) for financial support.
Author information
Authors and Affiliations
Contributions
A.R.O. designed the research. Q.Z. and D.Y.J. performed the calculations. Q.Z., D.Y.J., A.R.O. and C.G. interpreted data. A.O.L. and C.W.G. wrote the structure prediction code. Q.Z., D.Y.J., A.R.O. and C.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1948 kb)
Rights and permissions
About this article
Cite this article
Zhu, Q., Jung, D., Oganov, A. et al. Stability of xenon oxides at high pressures. Nature Chem 5, 61–65 (2013). https://doi.org/10.1038/nchem.1497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1497
This article is cited by
-
Structural Changes in Polycrystalline Indium Oxide After Irradiation with Xenon Ions
Refractories and Industrial Ceramics (2023)
-
Open questions on the high-pressure chemistry of the noble gases
Communications Chemistry (2022)
-
Materials under high pressure: a chemical perspective
Applied Physics A (2022)
-
The origin and fate of volatile elements on Earth revisited in light of noble gas data obtained from comet 67P/Churyumov-Gerasimenko
Scientific Reports (2020)
-
Chemistry under high pressure
Nature Reviews Chemistry (2020)