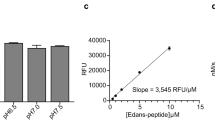
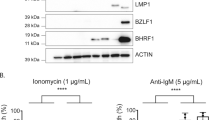
Abstract
Bryostatin is a unique lead in the development of potentially transformative therapies for cancer, Alzheimer's disease and the eradication of HIV/AIDS. However, the clinical use of bryostatin has been hampered by its limited supply, difficulties in accessing clinically relevant derivatives, and side effects. Here, we address these problems through the step-economical syntheses of seven members of a new family of designed bryostatin analogues using a highly convergent Prins-macrocyclization strategy. We also demonstrate for the first time that such analogues effectively induce latent HIV activation in vitro with potencies similar to or better than bryostatin. Significantly, these analogues are up to 1,000-fold more potent in inducing latent HIV expression than prostratin, the current clinical candidate for latent virus induction. This study provides the first demonstration that designed, synthetically accessible bryostatin analogues could serve as superior candidates for the eradication of HIV/AIDS through induction of latent viral reservoirs in conjunction with current antiretroviral therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fauci, A. S. et al. HIV vaccine research: the way forward. Science 321, 530–532 (2008).
Davey, R. T. Jr et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl Acad. Sci. USA 96, 15109–15114 (1999).
Carr, A. Toxicity of antiretroviral therapy and implications for drug development. Nature Rev. Drug Discov. 2, 624–634 (2003).
Mills, E. J. et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 3, 2039–2064 (2006).
Bangsberg, D. R. et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS 17, 1925–1932 (2003).
Ruff, C. T. et al. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J. Virol. 76, 9481–9492 (2002).
Bailey, J. R. et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80, 6441–6457 (2006).
Dinoso, J. B. et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 160, 9403–9408 (2009).
Coiras, M., Lopez-Huertas, M. R., Perez-Olmeda, M. & Alcami, J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nature Rev. 7, 798–812 (2009).
Finzi, D. et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature Med. 5, 512–517 (1999).
Richman, D. D. et al. The challenge of finding a cure for HIV infection. Science 323, 1304–1307 (2009).
Stellbrink, H-J. et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial). AIDS 16, 1479–1487 (2002).
Van Praag, R. M. E. et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD41 T cell depletion. J. Clin. Immunol. 21, 218–226 (2001).
Kulkosky, J. et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98, 3006–3015 (2001).
Korin, Y. D., Brooks, D. G., Brown, S., Korotzer, A. & Zack, J. A. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 76, 8118–8123 (2002).
Brow, S. J. & Hezarah, M. Methods of administering prostratin and structural analogs thereof. US patent no. 2009126949 (2009).
Wender, P. A., Kee, J-M. & Warrington, J. M. Practical synthesis of prostratin, DPP, and their analogs, adjuvant leads against latent HIV. Science 320, 649–652 (2008).
Pettit, G. R. et al. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 104, 6846–6848 (1982).
Kortmansky, J. & Schwartz, G. K. Bryostatin-1: a novel PKC inhibitor in clinical development. Cancer Invest. 21, 924–936 (2003).
Hale, K. J., Hummersone, M. G., Manaviazar, S. & Frigerio, M. The chemistry and biology of the bryostatin antitumour macrolides. Nat. Prod. Rep. 19, 413–453 (2002).
Etcheberrigaray, R. et al. Therapeutic effects of PKC activators in Alzheimer's disease transgenic mice. Proc. Natl Acad. Sci. USA 101, 11141–11146 (2004).
Kinter, A. L., Poli, G., Maury, W., Folks, T. M. & Fauci, A. S. Direct and cytokine-mediated activation of protein kinase C induces human immunodeficiency virus expression in chronically infected promonocytic cells. J. Virol. 64, 4306–4312 (1990).
Quatsha, K. A., Rudolph, C., Marme, D., Schachtele, C. & May, W. S. Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc. Natl Acad. Sci. USA 90, 4674–4676 (1993).
Mehla, R. et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One 5, e11160 (2010).
Barr, P. M. et al. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am. J. Hematol. 84, 484–487 (2009).
Schaufelberger, D. E. et al. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J. Nat. Prod. 54, 1265–1270 (1991).
Trindade-Silva, A. E., Lim-Fong, G. E., Sharp, K. H. & Haygood, M. G. Bryostatins: biological context and biotechnological prospects. Curr. Opin. Biotechnol. 21, 834–842 (2010).
Kageyama, M. et al. Synthesis of bryostatin 7. J. Am. Chem. Soc. 112, 7407–7408 (1990).
Evans, D. A. et al. Total synthesis of bryostatin 2. J. Am. Chem. Soc. 121, 7540–7552 (1999).
Ohmori, K. et al. Total synthesis of bryostatin 3. Angew. Chem. Int. Ed. 39, 2290–2294 (2000).
Trost, B. M. & Dong, G. Total synthesis of bryostatin 16 using atom-economical and chemoselective approaches. Nature 456, 485–488 (2008).
Keck, G. E., Poudel, Y. B., Cummins, T. J., Rudra, A. & Covel, J. A. Total synthesis of bryostatin 1. J. Am. Chem. Soc. 133, 744–747 (2011).
Wender, P. A. & Schrier, A. J. Total synthesis of bryostatin 9. J. Am. Chem. Soc. 133, 9228–9231 (2011).
Lu, Y., Woo, S. & Krische, M. J. Total synthesis of bryostatin 7 via C–C bond-forming hydrogenation. J. Am. Chem. Soc. 133, 13876–13879 (2011).
Wender, P. A. et al. Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proc. Natl Acad. Sci. USA 85, 7197–7201 (1988).
Wender, P. A. in Drug Discovery Research: New Frontiers in the Post-Genomic Era (ed. Huang, Z.) Ch. 6 (Wiley-VCH, 2007).
Wender, P. A., Loy, B. A. & Schrier, A. J. Translating nature's library: the bryostatins and function-oriented synthesis. Isr. J. Chem. 51, 453–472 (2011).
Wender, P. A., Verma, V. A., Paxton, T. J. & Pillow, T. H. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41, 40–49 (2008).
Wender, P. A. et al. Synthesis of the first members of a new class of biologically active bryostatin analogues. J. Am. Chem. Soc. 120, 4534–4535 (1998).
Wender, P. A. et al. The practical synthesis of a novel and highly potent analogue of bryostatin. J. Am. Chem. Soc. 124, 13648–13649 (2002).
Wender, P. A., DeChristopher, B. A. & Schrier, A. J. Efficient synthetic access to a new family of highly potent bryostatin analogues via a Prins-driven macrocyclization strategy. J. Am. Chem. Soc. 130, 6658–6659 (2008).
Wender, P. A. et al. Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity. Proc. Natl Acad. Sci. USA 108, 6721–6726 (2011).
Mackay, H. J. & Twelves, C. J. Targeting the protein kinase C family: are we there yet? Nature Rev. Cancer 7, 554–562 (2007).
DeChristopher, B. A., Fan, A. C., Felsher, D. W. & Wender, P. A. ‘Picolog’, a synthetically-available bryostatin analog, inhibits growth of MYC-induced lymphoma in vivo. Oncotarget 3, 58–66 (2012).
Khan, T. K., Nelson, T. J., Verma, V. A., Wender, P. A. & Alkon, D. L. A cellular model of Alzheimer's disease therapeutic efficacy: PKC activation reverses Aβ-induced biomarker abnormality on cultured fibroblasts. Neurobiol. Dis. 34, 332–339 (2009).
Crane, E. A. & Scheidt, K. A. Prins-type macrocyclizations as an efficient ring-closing strategy in natural product synthesis. Angew. Chem. Int. Ed. 49, 8316–8326 (2010).
Krasovskiy, A., Kopp, F. & Knochel, P. Soluble lanthanide salts (LnCl3·2LiCl) for the improved addition of organomagnesium reagents to carbonyl compounds. Angew. Chem. Int. Ed. 45, 497–500 (2006).
Marsden, M. D. & Zack, J. A. Establishment and maintenance of HIV latency: model systems and opportunities for intervention. Future Virol. 5, 97–109 (2010).
Jordan, A., Bisgrove, D. & Verdin, E. HIV reproducibly establishes a latent infection after acute infection in T cells in vitro. EMBO J. 22, 1868–1877 (2003).
Williams, S. A. et al. Prostratin antagonizes HIV latency by activating NF-κB. J. Biol. Chem. 279, 42008–42017 (2004).
Kovochich, M., Marsden, M. D. & Zack, J. A. Activation of latent HIV using drug-loaded nanoparticles. PLoS One 6, e18270 (2011).
Brooks, D. G. et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19, 413–423 (2003).
Acknowledgements
This research was supported by the National Institutes of Health (CA31845 to P.A.W., AI070010 to J.A.Z.) and the UCLA Center for AIDS Research (AI028697). Additional funding was provided by the ACS Division of Organic Chemistry Graduate Fellowship sponsored by Bristol-Myers Squibb, the Eli Lilly Organic Chemistry Graduate Fellowship, and the Stanford University Center for Molecular Analysis and Design.
Author information
Authors and Affiliations
Contributions
B.A.D., B.A.L., M.D.M., A.J.S., J.A.Z. and P.A.W. conceived and designed the experiments. B.A.D., B.A.L., M.D.M. and A.J.S. performed the experiments and analysed the data. B.A.L., M.D.M., A.J.S. and P.A.W. co-wrote the paper. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3410 kb)
Rights and permissions
About this article
Cite this article
DeChristopher, B., Loy, B., Marsden, M. et al. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nature Chem 4, 705–710 (2012). https://doi.org/10.1038/nchem.1395
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1395
This article is cited by
-
Occludin: a gatekeeper of brain Infection by HIV-1
Fluids and Barriers of the CNS (2023)
-
Characteristics and mechanisms of latency-reversing agents in the activation of the human immunodeficiency virus 1 reservoir
Archives of Virology (2023)
-
Engaging innate immunity in HIV-1 cure strategies
Nature Reviews Immunology (2022)
-
Latency reversal plus natural killer cells diminish HIV reservoir in vivo
Nature Communications (2022)
-
Mushrooms: an emerging resource for therapeutic terpenoids
3 Biotech (2019)