Abstract
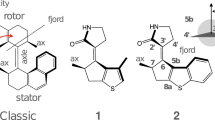
Light-driven molecular motors convert light into mechanical energy through excited-state reactions. Unidirectional rotary molecular motors based on chiral overcrowded alkenes operate through consecutive photochemical and thermal steps. The thermal (helix inverting) step has been optimized successfully through variations in molecular structure, but much less is known about the photochemical step, which provides power to the motor. Ultimately, controlling the efficiency of molecular motors requires a detailed picture of the molecular dynamics on the excited-state potential energy surface. Here, we characterize the primary events that follow photon absorption by a unidirectional molecular motor using ultrafast fluorescence up-conversion measurements with sub 50 fs time resolution. We observe an extraordinarily fast initial relaxation out of the Franck–Condon region that suggests a barrierless reaction coordinate. This fast molecular motion is shown to be accompanied by the excitation of coherent excited-state structural motion. The implications of these observations for manipulating motor efficiency are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
Balzani, V., Credi, A. & Venturi, M. Light powered molecular machines. Chem. Soc. Rev. 38, 1542–1550 (2009).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Koumura, N., Geertsema, E. M., van Gelder, M. B., Meetsma, A. & Feringa, B. L. Second generation light-driven molecular motors. Unidirectional rotation controlled by a single stereogenic center with near-perfect photoequilibria and acceleration of the speed of rotation by structural modification. J. Am. Chem. Soc. 124, 5037–5051 (2002).
Vicario, J., Meetsma, A. & Feringa, B. L. Controlling the speed of rotation in molecular motors. Dramatic acceleration of the rotary motion by structural modification. Chem. Comm. 5910–5912 (2005).
van Delden, R. A. et al. Unidirectional molecular motor on a gold surface. Nature 437, 1337–1340 (2005).
Pollard, M. M., Klok, M., Pijper, D. & Feringa, B. L. Rate acceleration of light-driven rotary molecular motors. Adv. Funct. Mater. 17, 718–729 (2007).
Klok, M. et al. MHz unidirectional rotation of molecular rotary motors. J. Am. Chem. Soc. 130, 10484–10485 (2008).
Klok, M., Browne, W. R. & Feringa, B. L. Kinetic analysis of the rotation rate of light-driven unidirectional molecular motors. Phys. Chem. Chem. Phys. 11, 9124–9131 (2009).
Augulis, R., Klok, M., Feringa, B. L. & van Loosdrecht, P. H. M. Light-driven rotary molecular motors: an ultrafast optical study. Phys. Status Solidi C 6, 181–184 (2009).
Kazaryan, A. et al. Understanding the dynamics behind the photoisomerization of a light-driven fluorene molecular rotary motor. J. Phys. Chem. A 114, 5058–5067 (2010).
Heisler, I. A., Kondo, M. & Meech, S. R. Reactive dynamics in confined liquids: ultrafast torsional dynamics of auramine O in nanoconfined water in aerosol OT reverse micelles. J. Phys. Chem. B 113, 1623–1631 (2009).
Glasbeek, M. & Zhang, H. Femtosecond studies of solvation and intramolecular configurational dynamics of fluorophores in liquid solution. Chem. Rev. 104, 1929–1954 (2004).
Morales, A. R., Belfield, K. D., Hales, J. M., Van Stryland, E. W. & Hagan, D. J. Synthesis of two-photon absorbing unsymmetrical fluorenyl-based chromophores. Chem. Mater. 18, 4972–4980 (2006).
Nakamura, T., Takeuchi, S., Suzuki, N. & Tahara, T. Revised steady-state fluorescence spectrum and nature of the reactive S(1) state of cis-stilbene in solution. Chem. Phys. Lett. 465, 212–215 (2008).
Mokhtari, A., Chebira, A. & Chesnoy, J. Subpicosecond fluorescence dynamics of dye molecules. J. Opt. Soc. Am. B 7, 1551–1557 (1990).
Hauer, J., Buckup, T. & Motzkus, M. Enhancement of molecular modes by electronically resonant multipulse excitation: further progress towards mode selective chemistry. J. Chem. Phys. 125, 061101 (2006).
Klok, M., Janssen, L., Browne, W. R. & Feringa, B. L. The influence of viscosity on the functioning of molecular motors. Faraday Discuss. 143, 319–334 (2009).
Pollard, W. T., Lee, S. Y. & Mathies, R. A. Wave packet theory of dynamic absorption-spectra in femtosecond pump-probe experiments. J. Chem. Phys. 92, 4012–4029 (1990).
Rubtsov, I. V. & Yoshihara, K. Vibrational coherence in electron donor–acceptor complexes. J. Phys. Chem. A 103, 10202–10212 (1999).
Sanchez-Galvez, A. et al. Ultrafast radiationless deactivation of organic dyes: evidence for a two-state two-mode pathway in polymethine cyanines. J. Am. Chem. Soc. 122, 2911–2924 (2000).
Hahn, S. & Stock, G. Quantum-mechanical modeling of the femtosecond isomerization in rhodopsin. J. Phys. Chem. B 104, 1146–1149 (2000).
Levine, B. G. & Martinez, T. J. Isomerization through conical intersections. Annu. Rev. Phys. Chem. 58, 613–634 (2007).
Myers, A. B. & Mathies, R. A. Excited-state torsional dynamics of cis-stilbene from resonance Raman intensities. J. Chem. Phys. 81, 1552–1558 (1984).
Ishii, K., Takeuchi, S. & Tahara, T. A 40-fs time-resolved absorption study on cis-stilbene in solution: observation of wavepacket motion on the reactive excited state. Chem. Phys. Lett. 398, 400–406 (2004).
Takeuchi, S. et al. Spectroscopic tracking of structural evolution in ultrafast stilbene photoisomerization. Science 322, 1073–1077 (2008).
Hauer, J., Buckup, T. & Motzkus, M. Quantum control spectroscopy of vibrational modes: comparison of control scenarios for ground and excited states in beta-carotene. Chem. Phys. 350, 220–229 (2008).
Prokhorenko, V. I. et al. Coherent control of retinal isomerization in bacteriorhodopsin. Science 313, 1257–1261 (2006).
Flores, S. C. & Batista, V. S. Model study of coherent-control of the femtosecond primary event of vision. J. Phys. Chem. B 108, 6745–6749 (2004).
Perez-Hernandez, G., Pelzer, A., Gonzalez, L. & Seideman, T. Biologically inspired molecular machines driven by light. Optimal control of a unidirectional rotor. New J. Phys. 12, 1–24 (2010).
Rhee, H. & Joo, T. Noncollinear phase matching in fluorescence upconversion. Opt. Lett. 30, 96–98 (2005).
Pollard, M. M., Meetsma, A. & Feringa, B. L. A redesign of light-driven rotary molecular motors. Org. Biomol. Chem. 6, 507–512 (2008).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EP/E010466), European Research Council (ERC) Starting Grant (279549; W.R.B.) and ERC Advanced Investigator Grant (227897; A.C., B.L.F.). J.C. was supported by a University of East Anglia studentship.
Author information
Authors and Affiliations
Contributions
S.R.M., B.L.F. and W.R.B. conceived and designed the experiments. J.C. and K.A. performed the time-resolved experiments, I.H. constructed the up-conversion apparatus, A.C. and B.L.F. designed and synthesized 1, J.C. and A.C. performed the steady-state electronic spectroscopy, J.C. and I.H. analysed the time-resolved data, A.C. performed the density functional theory calculations, W.R.B obtained and analysed the Raman data, S.R.M. wrote the paper and all the authors commented and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 757 kb)
Rights and permissions
About this article
Cite this article
Conyard, J., Addison, K., Heisler, I. et al. Ultrafast dynamics in the power stroke of a molecular rotary motor. Nature Chem 4, 547–551 (2012). https://doi.org/10.1038/nchem.1343
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1343
This article is cited by
-
Photo-responsive functional materials based on light-driven molecular motors
Light: Science & Applications (2024)
-
Ultrafast motion in a third generation photomolecular motor
Nature Communications (2023)
-
A photochemical method to evidence directional molecular motions
Nature Communications (2023)
-
Controlling dynamics in extended molecular frameworks
Nature Reviews Chemistry (2022)
-
Absolute excited state molecular geometries revealed by resonance Raman signals
Nature Communications (2022)