Abstract
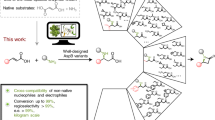
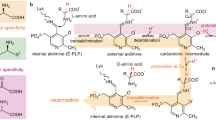
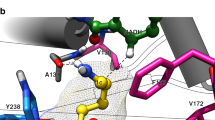
The redesign of enzymes to produce catalysts for a predefined transformation remains a major challenge in protein engineering. Here, we describe the structure-based engineering of methylaspartate ammonia lyase (which in nature catalyses the conversion of 3-methylaspartate to ammonia and 2-methylfumarate) to accept a variety of substituted amines and fumarates and catalyse the asymmetric synthesis of aspartic acid derivatives. We obtained two single-active-site mutants, one exhibiting a wide nucleophile scope including structurally diverse linear and cyclic alkylamines and one with broad electrophile scope including fumarate derivatives with alkyl, aryl, alkoxy, aryloxy, alkylthio and arylthio substituents at the C2 position. Both mutants have an enlarged active site that accommodates the new substrates while retaining the high stereo- and regioselectivity of the wild-type enzyme. As an example, we demonstrate a highly enantio- and diastereoselective synthesis of threo-3-benzyloxyaspartate (an important inhibitor of neuronal excitatory glutamate transporters in the brain).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sonke, T., Kaptein, B. & Schoemaker, H. E. Use of enzymes in the synthesis of amino acids, in Amino Acids, Peptides and Proteins in Organic Chemistry Vol. 1 (ed. Hughes, A. B.) 77–117 (Wiley-VCH, 2009).
Wohlgemuth, R. Biocatalysis—key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 21, 713–724 (2010).
Panke, S. & Wubbolts, M. Advances in biocatalytic synthesis of pharmaceutical intermediates. Curr. Opin. Chem. Biol. 9, 188–194 (2005).
Panke, S., Held, M. & Wubbolts, M. Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr. Opin. Biotechnol. 15, 272–279 (2004).
Turner, N. J. Ammonia lyases and aminomutases as biocatalysts for the synthesis of α-amino and β-amino acids. Curr. Opin. Chem. Biol. 15, 234–240 (2011).
Barker, H. A., Smyth, R. D., Wilson, R. M. & Weissbach, H. The purification and properties of β-methylaspartase. J. Biol. Chem. 234, 320–328 (1959).
Goda, S. K., Minton, N. P., Botting, N. P. & Gani, D. Cloning, sequencing, and expression in Escherichia coli of the Clostridium tetanomorphum gene encoding β-methylaspartase and characterization of the recombinant protein. Biochemistry 31, 10747–10756 (1992).
Raj, H. et al. Alteration of the diastereoselectivity of 3-methylaspartate ammonia lyase by using structure-based mutagenesis. ChemBioChem 10, 2236–2245 (2009).
Bright, H. J. Divalent metal activation of β-methylaspartase. The importance of ionic radius. Biochemistry 6, 1191–1203 (1967).
Bright, H. J. On the mechanism of divalent metal activation of β-methylaspartase. J. Biol. Chem. 240, 1198–1210 (1965).
Kato, Y. & Asano, Y. 3-Methylaspartate ammonia-lyase as a marker enzyme of the mesaconate pathway for (S)-glutamate fermentation in Enterobacteriaceae. Arch. Microbiol. 168, 457–463 (1997).
Asuncion, M., Blankenfeldt, W., Barlow, J. N., Gani, D. & Naismith, J. H. The structure of 3-methylaspartase from Clostridium tetanomorphum functions via the common enolase chemical step. J. Biol. Chem. 277, 8306–8311 (2002).
Levy, C. W. et al. Insights into enzyme evolution revealed by the structure of methylaspartate ammonia lyase. Structure 10, 105–113 (2002).
Bright, H. J., Ingraham, L. L. & Lundin, R. E. The mechanism of the methylaspartate ammonia–lyase reaction: deuterium exchange. Biochim. Biophys. Acta 81, 576–584 (1964).
Bright, H. The mechanism of the β-methylaspartase reaction. J. Biol. Chem. 239, 2307–2315 (1964).
Babbitt, P. C. et al. The enolase superfamily: a general strategy for enzyme-catalyzed abstraction of the α-protons of carboxylic acids. Biochemistry 35, 16489–16501 (1996).
Akhtar, M., Botting, N. P., Cohen, M. A. & Gani, D. Enantiospecific synthesis of 3-substituted aspartic acids via enzymic amination of substituted fumaric acids. Tetrahedron 43, 5899–5908 (1987).
Botting, N. P., Akhtar, M., Cohen, M. A. & Gani, D. Substrate specificity of the 3-methylaspartate ammonia-lyase reaction: observation of differential relative reaction rates for substrate-product pairs. Biochemistry 27, 2953–2955 (1988).
Gulzar, M. S., Akhtar, M. & Gani, D. Preparation of N-substituted aspartic acids via enantiospecific conjugate addition of N-nucleophiles to fumaric acids using methylaspartase: synthetic utility and mechanistic implications. J. Chem. Soc. Perkin Trans. 1, 649–656 (1997).
Lutz, S. & Bornscheuer, U. T. Protein Engineering Handbook. Vols 1–2 (Wiley-VCH, 2009).
Reetz, M. T., Bocola, M., Carballeira, J. D., Zha, D. & Vogel, A. Expanding the range of substrate acceptance of enzymes: combinatorial active-site saturation test. Angew. Chem. Int. Ed. 44, 4192–4196 (2005).
Morley, K. L. & Kazlauskas, R. J. Improving enzyme properties: when are closer mutations better? Trends Biotechnol. 23, 231–237 (2005).
Arnold, F. H. & Georgiou, G. (eds) Methods in Molecular Biology (Directed Enzyme Evolution) Vol. 230 (Humana Press, 2003).
Turner, N. J. Directed evolution drives the next generation of biocatalysts. Nature Chem. Biol. 5, 567–573 (2009).
Shimamoto, K. et al. Characterization of novel L-threo-β-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol. Pharmacol. 65, 1008–1015 (2004).
Shimamoto, K. Glutamate transporter blockers for elucidation of the function of excitatory neurotransmission systems. Chem. Rec. 8, 182–199 (2008).
Esslinger, C. S. et al. The substituted aspartate analogue L-β-threo-benzyl-aspartate preferentially inhibits the neuronal excitatory amino acid transporter EAAT3. Neuropharmacology 49, 850–861 (2005).
Bridges, R. J. & Esslinger, C. S. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol. Ther. 107, 271–285 (2005).
Mavencamp, T. L., Rhoderick, J. F., Bridges, R. J. & Esslinger, C. S. Synthesis and preliminary pharmacological evaluation of novel derivatives of L-β-threo-benzylaspartate as inhibitors of the neuronal glutamate transporter EAAT3. Bioorg. Med. Chem. 16, 7740–7748 (2008).
Shimamoto, K. et al. Syntheses of optically pure β-hydroxyaspartate derivatives as glutamate transporter blockers. Bioorg. Med. Chem. Lett. 10, 2407–2410 (2000).
Spengler, J., Pelay, M., Tulla-Puche, J. & Albericio, F. Synthesis of orthogonally protected L-threo-β-ethoxyasparagine. Amino Acids 39, 161–165 (2010).
LaBean, T. H. & Kauffman, S. A. Design of synthetic gene libraries encoding random sequence proteins with desired ensemble characteristics. Protein Sci. 2, 1249–1254 (1993).
Ho, S. N., Hunt, H. D., Horton, R. M., Pullen, J. K. & Pease, L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989).
Acknowledgements
The authors thank B. Wu, B. Kaptein and O. May for helpful discussions. This research was financially supported by VENI grant 700.54.401 and ECHO grant 700.59.042 (both to G.J.P.) from the Division of Chemical Sciences of the Netherlands Organisation of Scientific Research (NWO-CW), and by the Netherlands Ministry of Economic Affairs and the B-Basic partner organizations (www.b-basic.nl) through B-Basic, a public-private NWO–ACTS programme (ACTS, Advanced Chemical Technologies for Sustainability). Financial support from the Royal Netherlands Academy of Arts and Sciences (KNAW to W.S. and B.L.F.) is also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
H.R. performed mutagenesis, library screening and activity measurements. W.S. and J.d.V. synthesized starting substrates and reference compounds. H.R., W.S., M.d.V. and F.J.D. performed preparative biocatalysis. H.J.R. performed X-ray crystallography experiments. V.P.V. performed chiral HPLC. C.R.R. performed the molecular docking experiments. S.d.W., W.J.Q., A.M.W.H.T., B.L.F., D.B.J. and G.J.P. supervised scientific work. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1981 kb)
Rights and permissions
About this article
Cite this article
Raj, H., Szymański, W., de Villiers, J. et al. Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids. Nature Chem 4, 478–484 (2012). https://doi.org/10.1038/nchem.1338
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1338
This article is cited by
-
Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination
Nature Chemistry (2023)
-
Divergent synthesis of biologically active l-threo-β-hydroxyaspartates from common trans-oxazolidine dicarboxylate
Amino Acids (2022)
-
Chiral resolution of N-methyl-d,l-aspartic acid using a predicted N-demethylase from Chloroflexi bacterium 54-19
Systems Microbiology and Biomanufacturing (2022)
-
Structure-guided protein engineering of ammonia lyase for efficient synthesis of sterically bulky unnatural amino acids
Bioresources and Bioprocessing (2021)
-
Development of a versatile and efficient C–N lyase platform for asymmetric hydroamination via computational enzyme redesign
Nature Catalysis (2021)