Abstract
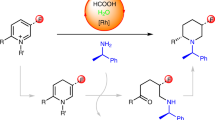
The enantioselective synthesis of nitrogen-containing heterocycles (N-heterocycles) represents a substantial chemical research effort and resonates across numerous disciplines, including the total synthesis of natural products and medicinal chemistry. In this Article, we describe the highly enantioselective palladium-catalysed decarboxylative allylic alkylation of readily available lactams to form 3,3-disubstituted pyrrolidinones, piperidinones, caprolactams and structurally related lactams. Given the prevalence of quaternary N-heterocycles in biologically active alkaloids and pharmaceutical agents, we envisage that our method will provide a synthetic entry into the de novo asymmetric synthesis of such structures. As an entry for these investigations we demonstrate how the described catalysis affords enantiopure quaternary lactams that intercept synthetic intermediates previously used in the synthesis of the Aspidosperma alkaloids quebrachamine and rhazinilam, but that were previously only available by chiral auxiliary approaches or as racemic mixtures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cordell, G. A. (ed.) in Alkaloids Vol. 69 (Academic Press, 2010).
Joule, J. A. & Mills, K. in Heterocyclic Chemistry, 5th edn (Wiley, 2010).
Anton, A. & Baird, B. R. in Kirk–Othmer Encyclopedia of Chemical Technology Vol. 19, 5th edn, 739–772 (Wiley, 2006).
Schlack, P. Polymerizable lactams. Pure Appl. Chem. 15, 507–523 (1967).
Kohan, M. I. (ed.) in Plastics (Interscience, 1973).
Groaning, M. D. & Meyers, A. I. Chiral Non-racemic bicyclic lactams. Auxiliary-based asymmetric reactions. Tetrahedron 56, 9843–9873 (2000).
Enders, D., Teschner, P., Raabe, G. & Runsink, J. Asymmetric electrophilic substitutions at the α-position of γ- and δ-lactams. Eur. J. Org. Chem. 4463–4477 (2001).
Amat, M., Lozano, O., Escolano, C., Molins, E. & Bosch, J. Enantioselective synthesis of 3,3-disubstituted piperidine derivatives by enolate dialkylation of phenylglycinol-derived oxazolopiperidone lactams. J. Org. Chem. 72, 4431–4439 (2007).
Trost, B. M. & Brennan, M. K. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis 3003–3025 (2009).
Badillo, J. J., Hanhan, N. V. & Franz, A. K. Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery. Curr. Opin. Drug Discov. Dev. 13, 758–776 (2010).
Zhou, F., Liu, Y.-L. & Zhou, J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381–1407 (2010).
Ohmatsu, K., Kiyokawa, M. & Ooi, T. Chiral 1,2,3-triazoliums as new cationic organic catalysts with anion-recognition ability: application to asymmetric alkylation of oxindoles. J. Am. Chem. Soc. 133, 1307–1309 (2011).
Franckevicius, V., Cuthbertson, J. D., Pickworth, M., Pugh, D. S. & Taylor, R. J. K. Asymmetric decarboxylative allylation of oxindoles. Org. Lett. 13, 4264–4267 (2011).
Jakubec, P., Helliwell, M. & Dixon, D. J. Cyclic imine nitro-Mannich/lactamization cascades: a direct stereoselective synthesis of multicyclic piperidinone derivatives. Org. Lett. 10, 4267–4270 (2008).
Moss, T. A., Alonso, B., Fenwick, D. R. & Dixon, D. J. Catalytic enantio- and diastereoselective alkylations with cyclic sulfamidates. Angew. Chem. Int. Ed. 49, 568–571 (2010).
Trost, B. M. Asymmetric allylic alkylation, an enabling methodology. J. Org. Chem. 69, 5813–5837 (2004).
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 47, 258–297 (2008).
Mohr, J. T. & Stoltz, B. M. Enantioselective Tsuji allylations. Chem. Asian J. 2, 1476–1491 (2007).
Weaver, J. D., Recio III, A., Grenning, A. J. & Tunge, J. A. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 111, 1846–1913 (2011).
Behenna, D. C. & Stoltz, B. M. The enantioselective Tsuji allylation. J. Am. Chem. Soc. 126, 15044–15045 (2004).
Mohr, J. T., Behenna, D. C., Harned, A. M. & Stoltz, B. M. Deracemization of quaternary stereocenters by Pd-catalyzed enantioconvergent decarboxylative allylation of racemic β-ketoesters. Angew. Chem. Int. Ed. 44, 6924–6927 (2005).
Seto, M., Roizen, J. L. & Stoltz, B. M. Catalytic enantioselective alkylation of substituted dioxanone enol ethers: ready access to C(α)-tetrasubstituted hydroxyketones, acids, and esters. Angew. Chem. Int. Ed. 47, 6873–6876 (2008).
Streuff, J., White, D. E., Virgil, S. C. & Stoltz, B. M. A palladium-catalysed enolate alkylation cascade for the formation of adjacent quaternary and tertiary stereocentres. Nature Chem. 2, 192–196 (2010).
McFadden, R. M. & Stoltz, B. M. The catalytic enantioselective, protecting group-free total synthesis of (+)-dichroanone. J. Am. Chem. Soc. 128, 7738–7739 (2006).
White, D. E., Stewart, I. C., Grubbs, R. H. & Stoltz, B. M. The catalytic asymmetric total synthesis of elatol. J. Am. Chem. Soc. 130, 810–811 (2008).
Enquist, J. A. Jr & Stoltz, B. M. The total synthesis of (–)-cyanthiwigin F via double catalytic enantioselective alkylation. Nature 453, 1228–1231 (2008).
Day, J. J. et al. The catalytic enantioselective total synthesis of (+)-liphagal. Angew. Chem. Int. Ed. 50, 6814–6818 (2011).
Trost, B. M. & Xu, J. Regio- and enantioselective Pd-catalyzed allylic alkylation of ketones through allyl enol carbonates. J. Am. Chem. Soc. 127, 2846–2847 (2005).
Trost, B. M. & Xu, J. Palladium-catalyzed asymmetric allylic α-alkylation of acyclic ketones. J. Am. Chem. Soc. 127, 17180–17181 (2005).
Trost, B. M., Bream, R. N. & Xu, J. Asymmetric allylic alkylation of cyclic vinylogous esters and thioesters by Pd-catalyzed decarboxylation of enol carbonate and β-ketoester substrates. Angew. Chem. Int. Ed. 45, 3109–3112 (2006).
Trost, B. M., Xu, J. & Reichle, M. Enantioselective synthesis of α-tertiary hydroxyaldehydes by palladium-catalyzed asymmetric allylic alkylation of enolates. J. Am. Chem. Soc. 129, 282–283 (2007).
Trost, B. M., Xu, J. & Schmidt, T. Palladium-catalyzed decarboxylative asymmetric allylic alkylation of enol carbonates. J. Am. Chem. Soc. 131, 18343–18357 (2009).
Nakamura, M., Hajra, A., Endo, K. & Nakamura, E. Synthesis of chiral α-fluoroketones through catalytic enantioselective decarboxylation. Angew. Chem. Int. Ed. 44, 7248–7251 (2005).
Burger, E. C., Barron, B. R. & Tunge, J. A. Catalytic asymmetric synthesis of cyclic α-allylated α-fluoroketones. Synlett. 2824–2826 (2006).
Bélanger, É., Cantin, K., Messe, O., Tremblay, M. & Paquin, J-F. Enantioselective Pd-catalyzed allylation reaction of fluorinated silyl enol ethers. J. Am. Chem. Soc. 129, 1034–1035 (2007).
Schulz, S. R. & Blechert, S. Palladium-catalyzed synthesis of substituted cycloheptane-1,4-diones by an asymmetric ring-expanding allylation (AREA). Angew. Chem. Int. Ed. 46, 3966–3970 (2007).
McDougal, N. T., Virgil, S. C. & Stoltz, B. M. High-throughput screening of the asymmetric decarboxylative alkylation reaction of enolate-stabilized enol carbonates. Synlett. 1712–1716 (2010).
Helmchen, G. & Pfaltz, A. Phosphinooxazolines—a new class of versatile, modular P,N-ligands for asymmetric catalysis. Acc. Chem. Res. 33, 336–345 (2000).
Tani, K., Behenna, D. C., McFadden, R. M. & Stoltz, B. M. A facile and modular synthesis of phosphinooxazoline ligands. Org. Lett. 9, 2529–2531 (2007).
McDougal, N. T., Streuff, J., Mukherjee, H., Virgil, S. C. & Stoltz, B. M. Rapid synthesis of an electron-deficient t-BuPHOX ligand: cross-coupling of aryl bromides with secondary phosphine oxides. Tetrahedron Lett. 51, 5550–5554 (2010).
Edler, M. C. et al. Demonstration of microtubule-like structures formed with (–)-rhazinilam from purified tubulin outside of cells and a simple tubulin-based assay for evaluation of analog activity. Arch. Biochem. Biophys. 487, 98–104 (2009).
Magnus, P. & Rainey, T. Concise synthesis of (±)-rhazinilam. Tetrahedron 57, 8647–8651 (2001).
Acknowledgements
This publication is based on work supported by award from the King Abdullah University of Science and Technology (KAUST; no. KUS-11-006-02). The authors thank NIH-NIGMS (R01GM080269-01 and a postdoctoral fellowship to D.E.W.), the Gordon and Betty Moore Foundation, Amgen, Abbott, Boehringer Ingelheim and Caltech for financial support. T.Y. acknowledges the Japan Society for the Promotion of Science for a predoctoral fellowship.
Author information
Authors and Affiliations
Contributions
D.C.B., Y.L., T.Y. and J.K. planned and carried out the experimental work. D.C.B., T.Y., D.E.W. and S.C.V. took part in the initial reaction development and screening experiments. B.M.S. conceived, initiated and directed the project and wrote the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 11489 kb)
Rights and permissions
About this article
Cite this article
Behenna, D., Liu, Y., Yurino, T. et al. Enantioselective construction of quaternary N-heterocycles by palladium-catalysed decarboxylative allylic alkylation of lactams. Nature Chem 4, 130–133 (2012). https://doi.org/10.1038/nchem.1222
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1222
This article is cited by
-
Transition-metal-free silylboronate-mediated cross-couplings of organic fluorides with amines
Nature Communications (2023)
-
Enantioselective synthesis of γ-butenolides through Pd-catalysed C5-selective allylation of siloxyfurans
Nature Synthesis (2022)