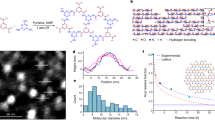
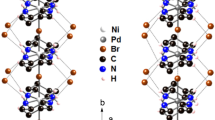
Abstract
One-dimensional metal wires are valuable materials because of their optical and electronic anisotropy, and they have potential utility in devices such as photovoltaic cells and molecular sensors. However, despite more than a century of research, only a few examples exist of well-defined one-dimensional (1D) metal wires that allow for the rational variation of conductivity. Herein we describe the first examples of 1D molecular wires supported by Pd–Pd bonds, the thin-film conductive properties of which can be altered by controlled molecular changes. Wires based on Pd(III) give semiconducting films with a modifiable bandgap, whereas wires based on Pd(2.5) give films that display metallic conductivity above 200 K: a metallic state has not been reported previously for any polymer composed of 1D metal wires. The wires are infinite in the solid state and maintain 1D structures in solution with lengths of up to 750 nm. Solution stability enables thin film coating, a requisite for device fabrication using molecular wires.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bera, J. K. & Dunbar, K. R. Chain compounds based on transition metal backbones: new life for an old topic. Angew. Chem. Int. Ed. 41, 4453–4457 (2002).
Hofmann, K. A. & Bugge, G. Platinblau. Chem. Ber. 41, 312–314 (1908).
Barton, J., Rabinowitz, H., Szalda, D. & Lippard, S. Synthesis and crystal structure of cis-diammineplatinum .alpha.-pyridone blue. J. Am. Chem. Soc. 99, 2827–2829 (1977).
Lippard, S. New chemistry of an old molecule: cis-[Pt(NH3)2Cl2]. Science 218, 1075–1082 (1982).
Lippert, B. Impact of cisplatin on the recent development of Pt coordination chemistry: a case study. Coord. Chem. Rev. 182, 263–295 (1999).
Sigal, I. S. & Gray, H. B. Characterization of cationic rhodium isocyanide oligomers in aqueous solutions. J. Am. Chem. Soc. 203, 2220–2225 (1981).
Tejel, C. et al. Discrete mixed-valence metal chains: iridium pyridonate blues. Angew. Chem. Int. Ed. 40, 4084–4086 (2001).
Miller, J. S. & Epstein, A. J. One-dimensional inorganic complexes. Prog. Inorg. Chem. 20, 1–151 (1976).
Masciocchi, N., Sironi, A., Chardon-Noblat, S. & Deronzier, A. X-ray powder diffraction study of organometallic polymers: [Ru(L)(CO)2)]n (L = 2,2′-bipyridine or 1,10-phenanthroline). Organometallics 21, 4009–4012 (2002).
Krogmann, K. Planar complexes containing metal–metal bonds. Angew. Chem. Int. Ed. Engl. 8, 35–42 (1969).
Finniss, G. M., Canadell, E., Campana, C. & Dunbar, K. R. Unprecedented conversion of a compound with metal–metal bonding into a solvated molecular wire. Angew. Chem. Int. Ed. Engl. 35, 2772–2774 (1996).
Cotton, F., Dikarev, E. & Petrukhina, M. Studies of tetrakis(trifluoroacetate)dirhodium. Part 4. Solventless synthesis of Rh2(O2CCF3)2(CO)4 combined with Rh2(O2CCF3)4, a compound with infinite chains of rhodium atoms. J. Organomet. Chem. 596, 130–135 (2000).
Cotton, F., Dikarev, E. & Petrukhina, M. cis-Di(μ-trifluoroacetate)dirhodium tetracarbonyl: structure and chemistry. J. Chem. Soc. Dalton Trans. 4241–4243 (2000).
Lafolet, F. et al. Electrochemical fabrication and characterization of thin films of redox-active molecular wires based on extended Rh–Rh bonded chains. Dalton Trans. 2149–2156 (2008).
Pruchnik, F. P. et al. Rhodium wires based on binuclear acetate-bridged complexes. Inorg. Chem. Commun. 4, 19–22 (2001).
Swager, T. The molecular wire approach to sensory signal amplification. Acc. Chem. Res. 31, 201–207 (1998).
Frampton, M. J. & Anderson, H. L. Insulated molecular wires. Angew. Chem. Int. Ed. 46, 1028–1064 (2007).
Cheng, Y-J., Yang, S-H. & Hsu, C-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 109, 5868–5923 (2009).
Habas, S. E., Platt, H. A. A., van Hest, M. F. A. M. & Ginley, D. S. Low-cost inorganic solar cells: from ink to printed device. Chem. Rev. 110, 6571–6594 (2010).
Carroll, R. & Gorman, C. The genesis of molecular electronics. Angew. Chem. Int. Ed. 41, 4378–4400 (2002).
Georgiev, V. P & McGrady, J. E. Influence of low-symmetry distortions on electron transport through metal atom chains: when is a molecular wire really ‘broken’? J. Am. Chem. Soc. 133, 12590–12599 (2011).
Roncali, J. Synthetic principles for bandgap control in linear π-conjugated systems. Chem. Rev. 97, 173–205 (1997).
Jang, K. et al. One-dimensional organometallic molecular wires via assembly of Rh(CO)2Cl(amine): chemical control of interchain distances and optical properties. J. Am. Chem. Soc. 131, 12046–12047 (2009).
Powers, D. C. & Ritter, T. Bimetallic Pd(III) complexes in palladium-catalysed carbon–heteroam bond formation. Nature Chem. 1, 302–309 (2009).
Bercaw, J. E. et al. Electronic structures of PdII dimers. Inorg. Chem. 49, 1801–1810 (2010).
Matsumoto, K. et al. Syntheses, crystal structures, and electronic, ESR, and X-ray photoelectron spectra of acetamidate- and 2-fluoroacetamidate-bridged mixed-valent octanuclear platinum blues. J. Am. Chem. Soc. 114, 8110–8118 (1992).
O'Halloran, T., Roberts, M. & Lippard, S. Correlation between metal–metal distances and optical spectroscopy in the platinum blues: synthesis, crystal structure, and electronic spectrum of ethylenediamine platinum .alpha.-pyridone blue. J. Am. Chem. Soc. 106, 6427–6428 (1984).
Nocera, D. G. Chemistry of multielectron excited states. Acc. Chem. Res. 28, 209–217 (1995).
Berry, J. et al. A fractional bond order of 1/2 in Pd5+2–formamidinate species; the value of very high-field EPR spectra. J. Am. Chem. Soc. 129, 1393–1401 (2007).
Cotton, F. A., Matusz, M., Poli, R. & Feng, X. Dinuclear formamidinato complexes of nickel and palladium. J. Am. Chem. Soc. 110, 1144–1154 (1988).
Peierls, R. E. Quantum Theory of Solids (Oxford University Press, 1955).
Acknowledgements
We thank T.A. Betley, A. Cohen and D.G. Nocera for discussions, S-L. Zheng and P. Müller for X-ray crystallographic analysis, T. Cook for help with NIR spectroscopy, R.A. Cabanas, S. Fraden and C. Schatz for assistance with light scattering, the Air Force Office of Scientific Research (FA9550-10-1-0170) and National Science Foundation (CHE-0952753) for funding and the Department of Energy Office of Science Graduate Fellowship for a graduate fellowship for M.G.C.
Author information
Authors and Affiliations
Contributions
M.G.C., D.C.P., J.R. and T.R. conceived and designed the experiments, M.G.C., D.C.P., J.R., M.J.G. and P.X. performed the experiments, M.G.C. and E.L. carried out the theoretical calculations and M.G.C., D.C.P., J.R. and T.R. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 9506 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 25 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 25 kb)
Supplementary information
Crystallographic data for compound 8 at 100 K (CIF 27 kb)
Supplementary information
Crystallographic data for compound 8 at 130 K (CIF 26 kb)
Supplementary information
Crystallographic data for compound 8 at 160 K (CIF 26 kb)
Supplementary information
Crystallographic data for compound 8 at 190 K (CIF 26 kb)
Supplementary information
Crystallographic data for compound 8 at 220 K (CIF 26 kb)
Supplementary information
Crystallographic data for compound 8 at 250 K (CIF 27 kb)
Rights and permissions
About this article
Cite this article
Campbell, M., Powers, D., Raynaud, J. et al. Synthesis and structure of solution-stable one-dimensional palladium wires. Nature Chem 3, 949–953 (2011). https://doi.org/10.1038/nchem.1197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1197
This article is cited by
-
Design of Concentric Cylindrical Surrounding Double-Gate (CSDG) MOSFETs – A Fabrication Perspective in Nanoscale Regime
Silicon (2023)
-
Structural, optical, and electrical properties of phase-controlled cesium lead iodide nanowires
Nano Research (2017)
-
Bandgap engineering of single layer graphene by randomly distributed nanoparticles
Journal of Materials Science: Materials in Electronics (2016)
-
Multinuclear metal-binding ability of a carotene
Nature Communications (2015)
-
Suppressed phase transition and giant ionic conductivity in La2Mo2O9 nanowires
Nature Communications (2015)