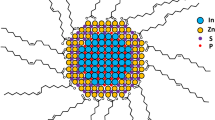
Abstract
Quantum dots are highly fluorescent and photostable, making them excellent tools for imaging. When using these quantum dots in cells and animals, however, intracellular biothiols (such as glutathione and cysteine) can degrade the quantum dot monolayer, compromising function. Here, we describe a label-free method to quantify the intracellular stability of monolayers on quantum dot surfaces that couples laser desorption/ionization mass spectrometry with inductively coupled plasma mass spectrometry. Using this new approach we have demonstrated that quantum dot monolayer stability is correlated with both quantum dot particle size and monolayer structure, with appropriate choice of both particle size and ligand structure required for intracellular stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Mater. 4, 435–446 (2005).
Zrazhevskiy, P., Sena, M. & Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 39, 4326–4354 (2010).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Chan, W. C. W. & Nie, S. M. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281, 2016–2018 (1998).
Smith, A. M., Duan, H. W., Mohs, A. M. & Nie, S. M. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 60, 1226–1240 (2008).
Alivisatos, A. P., Gu, W. W. & Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 7, 55–76 (2005).
Dahan, M. et al. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 302, 442–445 (2003).
Lidke, D. S. et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nature Biotechnol. 22, 198–203 (2004).
Choi, H. S. et al. Renal clearance of quantum dots. Nature Biotechnol. 25, 1165–1170 (2007).
Smith, A. M., Duan, H., Rhyner, M. N., Ruan, G. & Nie, S. A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys. Chem. Chem. Phys. 8, 3895–3903 (2006).
Susumu, K. et al. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J. Am. Chem. Soc. 129, 13987–13996 (2007).
Liu, W. et al. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc. 130, 1274–1284 (2008).
Stewart, M. H. et al. Multidentate poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J. Am. Chem. Soc. 132, 9804–9813 (2010).
Li, D. et al. Glutathione-mediated release of functional plasmid DNA from positively charged quantum dots. Biomaterials 29, 2776–2782 (2008).
Han, G. et al. Controlled recovery of the transcription of nanoparticle-bound DNA by intracellular concentrations of glutathione. Bioconjugate Chem. 16, 1356–1359 (2005).
Hong, R. et al. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 128, 1078–1079 (2006).
Chompoosor, A., Han, G. & Rotello, V. M. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjug. Chem. 19, 1342–1345 (2008).
Pace, H. E., Lesher, E. K. & Ranville, J. F. Influence of stability on the acute toxicity of CdSe/ZnS nanocrystals to Daphnia magna. Environ. Toxicol. Chem. 29, 1338–1344 (2010).
Li-Shishido, S., Watanabe, T. M., Tada, H., Higuchi, H. & Ohuchi, N. Reduction in nonfluorescence state of quantum dots on an immunofluorescence staining. Biochem. Biophys. Res. Commun. 351, 7–13 (2006).
Zhang, F., Ali, Z., Amin, F., Riedinger, A. & Parak, W. In vitro and intracellular sensing by using the photoluminescence of quantum dots. Anal. Bioanal. Chem. 397, 935–942 (2010).
Park, C. & Yoon, T. H. l-Cysteine-induced photoluminescence enhancement of CdSe/ZnSe quantum dots in aqueous solution. Colloids Surf. B 75, 472–477 (2010).
Zhu, Z. J., Ghosh, P. S., Miranda, O. R., Vachet, R. W. & Rotello, V. M. Multiplexed screening of cellular uptake of gold nanoparticles using laser desorption/ionization mass spectrometry. J. Am. Chem. Soc. 130, 14139–14143 (2008).
Zhu, Z. J., Rotello, V. M. & Vachet, R. W. Engineered nanoparticle surfaces for improved mass spectrometric analyses. Analyst 134, 2183–2188 (2009).
Yan, B. et al. Laser desorption/ionization mass spectrometry analysis of monolayer-protected gold nanoparticles. Anal. Bioanal. Chem. 396, 1025–1035 (2010).
Al-Hajaj, N. A. et al. Short ligands affect modes of QD uptake and elimination in human cells. ACS Nano 5, 4909–4918 (2011).
Pisoni, R. L., Acker, T. L., Lisowski, K. M., Lemons, R. M. & Thoene, J. G. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J. Cell Biol. 110, 327–335 (1990).
Pisoni, R. L., Park, G. Y., Velilla, V. Q. & Thoene, J. G. Detection and characterization of a transport-system mediating cysteamine entry into human fibroblast lysosomes—specificity for aminoethylthiol and aminoethylsulfide derivatives. J. Biol. Chem. 270, 1179–1184 (1995).
Krepela, E., Prochazka, J. & Karova, B. Regulation of cathepsin B activity by cysteine and related thiols. Biol. Chem. 380, 541–551 (1999).
Chithrani, B. D., Ghazani, A. A. & Chan, W. C. W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6, 662–668 (2006).
Hostetler, M. J. et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 14, 17–30 (1998).
Hill, H. D., Millstone, J. E., Banholzer, M. J. & Mirkin, C. A. The role radius of rurvature plays in thiolated oligonucleotide loading on gold nanoparticles. ACS Nano 3, 418–424 (2009).
Olmos-Asar, J. A., Rapallo, A. & Mariscal, M. M. Development of a semiempirical potential for simulations of thiol–gold interfaces. Application to thiol-protected gold nanoparticles. Phys. Chem. Chem. Phys. 13, 6500–6506 (2011).
Mei, B. C. et al. Effects of ligand coordination number and surface curvature on the stability of gold nanoparticles in aqueous solutions. Langmuir 25, 10604–10611 (2009).
Puri, R. N. & Meister, A. Transport of glutathione, as γ-glutamylcysteinylglycyl ester, into liver and kidney. Proc. Natl Acad. Sci. USA 80, 5258–5260 (1983).
Yeh, Y.-C. et al. Synthesis of cationic quantum dots via a two-step ligand exchange process. Chem. Commun. 47, 3069–3071 (2011).
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health (grants R21 ES017871-01 and GM077173-05) and through the Center for Hierarchical Manufacturing (National Science Foundation grant DMI-0531171). The authors thank J.F. Tyson for access to the ICP-MS instrumentation, and B. Creran for assistance with transmission electron microscopy.
Author information
Authors and Affiliations
Contributions
Z.J.Z., V.M.R. and R.W.V. conceived and designed the experiments. Z.J.Z., Y.C.Y., R.T., B.Y. and J.T. performed the experiments. All authors analysed and discussed the data. Z.J.Z. wrote the manuscript, with revisions by V.M.R. and R.W.V.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2197 kb)
Rights and permissions
About this article
Cite this article
Zhu, ZJ., Yeh, YC., Tang, R. et al. Stability of quantum dots in live cells. Nature Chem 3, 963–968 (2011). https://doi.org/10.1038/nchem.1177
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1177
This article is cited by
-
Intracellular redox potential-driven live-cell synthesis of CdSe quantum dots in Staphylococcus aureus
Science China Chemistry (2024)
-
Gram scale synthesis of QD450 core–shell quantum dots for cellular imaging and sorting
Applied Nanoscience (2020)
-
An in vitro assay and artificial intelligence approach to determine rate constants of nanomaterial-cell interactions
Scientific Reports (2019)
-
Living cell synthesis of CdSe quantum dots: Manipulation based on the transformation mechanism of intracellular Se-precursors
Nano Research (2018)
-
Singlet Oxygen Generating Properties of Different Sizes of Charged Graphene Quantum Dot Nanoconjugates with a Positively Charged Phthalocyanine
Journal of Fluorescence (2018)