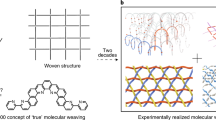
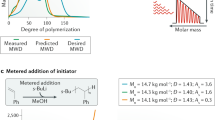
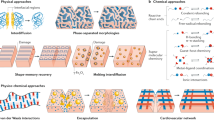
Abstract
Fundamental polymer science is undergoing a profound transformation. As a result of recent progress in macromolecular chemistry and physics, synthetic polymer chains are becoming much more than just the modest building blocks of traditional 'plastics'. Promising options for controlling the primary and secondary structures of synthetic polymers have been proposed and, therefore, similarly to biopolymers, synthetic macromolecules may now be exploited as discrete objects with carefully engineered structures and functions. Although it is not possible today to reach the high level of complexity found in biomaterials, these new chemical possibilities open interesting avenues for applications in microelectronics, photovoltaics, catalysis and biotechnology. Here, we describe in detail these recent advances in macromolecular science and emphasize the possible emergence of technologies based on single-chain devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Forrest, S. R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428, 911–918 (2004).
Langer, R. & Tirrell, D. A. Designing materials for biology and medicine. Nature 428, 487–492 (2004).
Stuart, M. A. C. et al. Emerging applications of stimuli-responsive polymer materials. Nature Mater. 9, 101–113 (2010).
Tomalia, D. A., Naylor, A. M. & Goddard, W. A. Starburst dendrimers - molecular-level control of size, shape, surface-chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Engl. 29, 138–175 (1990).
Hecht, S. & Fréchet, J. M. J. Dendritic encapsulation of function: applying nature's site isolation principle from biomimetics to materials science. Angew. Chem. Int. Ed. 40, 74–91 (2001).
Ouchi, M., Terashima, T. & Sawamoto, M. Transition metal-catalyzed living radical polymerization: toward perfection in catalysis and precision polymer synthesis. Chem. Rev. 109, 4963–5050 (2009).
Matyjaszewski, K. & Tsarevsky, N. V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nature Chem. 1, 276–288 (2009).
van Hest, J. C. M. & Tirrell, D. A. Protein-based materials, toward a new level of structural control. Chem. Commun. 1897–1904 (2001).
Hawker, C. J. & Wooley, K. L. The convergence of synthetic organic and polymer chemistries. Science 309, 1200–1205 (2005).
Badi, N. & Lutz, J.-F. Sequence control in polymer synthesis. Chem. Soc. Rev. 38, 3383–3390 (2009).
Brudno, Y. & Liu, D. R. Recent progress toward the templated synthesis and directed evolution of sequence-defined synthetic polymers. Chem. Biol. 16, 265–276 (2009).
Yamamoto, T. & Tezuka, Y. Topological polymer chemistry: a cyclic approach toward novel polymer properties and functions. Polym. Chem. 2, 1930–1941 (2011).
Hibi, Y. et al. Design of AB divinyl “template monomers” toward alternating sequence control in metal-catalyzed living radical polymerization. Polym. Chem. 2, 341–347 (2011).
Hibi, Y., Ouchi, M. & Sawamoto, M. Sequence-regulated radical polymerization with a metal-templated monomer: repetitive ABA sequence by double cyclopolymerization. Angew. Chem. Int. Ed. 50, 7434–7437 (2011).
Satoh, K. et al. Sequence-regulated vinyl copolymers by metal-catalysed step-growth radical polymerization. Nature Commun. 1, 6 (2010).
Seitz, M. E. et al. Nanoscale morphology in precisely sequenced poly(ethylene-co-acrylic acid) zinc ionomers. J. Am. Chem. Soc. 132, 8165–8174 (2010).
Pfeifer, S., Zarafshani, Z., Badi, N. & Lutz, J.-F. Liquid-phase synthesis of block copolymers containing sequence-ordered segments. J. Am. Chem. Soc. 131, 9195–9197 (2009).
Yu, T. B., Bai, J. Z. & Guan, Z. B. Cycloaddition-promoted self-assembly of a polymer into well-defined beta sheets and hierarchical nanofibrils. Angew. Chem. Int. Ed. 48, 1097–1101 (2009).
Li, J., Stayshich, R. M. & Meyer, T. Y. Exploiting sequence to control the hydrolysis behavior of biodegradable PLGA copolymers. J. Am. Chem. Soc. 133, 6910–6913 (2011).
Thomas, C. M. & Lutz, J.-F. Precision synthesis of biodegradable polymers. Angew. Chem. Int. Ed. 50, 9244–9246 (2011).
Tong, X., Guo, B.-h. & Huang, Y. Toward the synthesis of sequence-controlled vinyl copolymers. Chem. Commun. 47, 1455–1457 (2011).
Datta, B. & Schuster, G. B. DNA-directed synthesis of aniline and 4-aminobiphenyl oligomers: programmed transfer of sequence information to a conjoined polymer nanowire. J. Am. Chem. Soc. 130, 2965–2973 (2008).
Lin, N.-T. et al. From polynorbornene to the complementary polynorbornene by replication. Angew. Chem. Int. Ed. 46, 4481–4485 (2007).
Lo, P. K. & Sleiman, H. F. Nucleobase-templated polymerization: copying the chain length and polydispersity of living polymers into conjugated polymers. J. Am. Chem. Soc. 131, 4182–4183 (2009).
Ida, S., Terashima, T., Ouchi, M. & Sawamoto, M. Selective radical addition with a designed heterobifunctional halide: a primary study toward sequence-controlled polymerization upon template effect. J. Am. Chem. Soc. 131, 10808–10809 (2009).
Ida, S., Ouchi, M. & Sawamoto, M. Template-assisted selective radical addition toward sequence-regulated polymerization: lariat capture of target monomer by template initiator. J. Am. Chem. Soc. 132, 14748–14750 (2010).
Ida, S., Terashima, T., Ouchi, M. & Sawamoto, M. Selective single monomer addition in living cationic polymerization: sequential double end-functionalization in combination with capping agent. J. Polym. Sci. A 48, 3375–3381 (2010).
Pfeifer, S. & Lutz, J.-F. A facile procedure for controlling monomer sequence distribution in radical chain polymerizations. J. Am. Chem. Soc. 129, 9542–9543 (2007).
Pfeifer, S. & Lutz, J.-F. Development of a library of N-substituted maleimides for the local functionalization of linear polymer chains. Chem. Eur. J. 14, 10949–10957 (2008).
Lutz, J.-F., Schmidt, B. V. K. J. & Pfeifer, S. Tailored polymer microstructures prepared by atom transfer radical copolymerization of styrene and N-substituted maleimides. Macromol. Rapid Commun. 32, 127–135 (2011).
Kramer, J. W. et al. Polymerization of enantiopure monomers using syndiospecific catalysts: a new approach to sequence control in polymer synthesis. J. Am. Chem. Soc. 131, 16042–16044 (2009).
Lutz, J.-F. Polymer chemistry: a controlled sequence of events. Nature Chem. 2, 84–85 (2010).
Satoh, K., Matsuda, M., Nagai, K. & Kamigaito, M. AAB-sequence living radical chain copolymerization of naturally occurring limonene with maleimide: an end-to-end sequence-regulated copolymer. J. Am. Chem. Soc. 132, 10003–10005 (2010).
Connor, R. E. & Tirrell, D. A. Non-canonical amino acids in protein polymer design. Polym. Rev. 47, 9–28 (2007).
Hill, D. J. et al. A field guide to foldamers. Chem. Rev. 101, 3893–4012 (2001).
Guichard, G. & Huc, I. Synthetic foldamers. Chem. Commun. 47, 5933–5941 (2011).
Hecht, S. Construction with macromolecules. Mater. Today 8, 48–55 (2005).
Ghosh, S. & Ramakrishnan, S. Aromatic donor–acceptor charge-transfer and metal-ion-complexation-assisted folding of a synthetic polymer. Angew. Chem. Int. Ed. 43, 3264–3268 (2004).
van Gorp, J. J., Vekemans, J. & Meijer, E. W. Facile synthesis of a chiral polymeric helix; folding by intramolecular hydrogen bonding. Chem. Commun. 60–61 (2004).
Kumaki, J. et al. Molecular weight recognition in the multiple-stranded helix of a synthetic polymer without specific monomer–monomer interaction. J. Am. Chem. Soc. 130, 6373–6380 (2008).
Yashima, E. et al. Helical polymers: synthesis, structures, and functions. Chem. Rev. 109, 6102–6211 (2009).
Cornelissen, J. et al. β-helical polymers from isocyanopeptides. Science 293, 676–680 (2001).
Harth, E. et al. A facile approach to architecturally defined nanoparticles via intramolecular chain collapse. J. Am. Chem. Soc. 124, 8653–8660 (2002).
Zhou, Y., Jiang, K., Song, Q. & Liu, S. Thermo-induced formation of unimolecular and multimolecular micelles from novel double hydrophilic multiblock copolymers of N,N-dimethylacrylamide and N-isopropylacrylamide. Langmuir 23, 13076–13084 (2007).
Foster, E. J., Berda, E. B. & Meijer, E. W. Metastable supramolecular polymer nanoparticles via intramolecular collapse of single polymer chains. J. Am. Chem. Soc. 131, 6964–6966 (2009).
Mes, T., van der Weegen, R., Palmans, A. R. A. & Meijer, E. W. Single-chain polymeric nanoparticles by stepwise folding. Angew. Chem. Int. Ed. 50, 5085–5089 (2011).
Schmidt, B. V. K. J., Fechler, N., Falkenhagen, J. & Lutz, J.-F. Controlled folding of synthetic polymer chains through the formation of positionable covalent bridges. Nature Chem. 3, 234–238 (2011).
Terashima, T. et al. Single-chain folding of polymers for catalytic systems in water. J. Am. Chem. Soc. 133, 4742–4745 (2011).
Giuseppone, N. & Lutz, J.-F. Materials chemistry: catalytic accordions. Nature 473, 40–41 (2011).
Granick, S. et al. Single-molecule methods in polymer science. J. Polym. Sci. B 48, 2542–2543 (2010).
Kumaki, J., Nishikawa, Y. & Hashimoto, T. Visualization of single-chain conformations of a synthetic polymer with atomic force microscopy. J. Am. Chem. Soc. 118, 3321–3322 (1996).
Gromer, A., Rawiso, M. & Maaloum, M. Visualization of hydrophobic polyelectrolytes using atomic force microscopy in solution. Langmuir 24, 8950–8953 (2008).
Kiriy, A. et al. Cascade of coil-globule conformational transitions of single flexible polyelectrolyte molecules in poor solvent. J. Am. Chem. Soc. 124, 13454–13462 (2002).
Zhang, B. Z. et al. The largest synthetic structure with molecular precision: towards a molecular object. Angew. Chem. Int. Ed. 50, 737–740 (2011).
Sheiko, S. S., Sumerlin, B. S. & Matyjaszewski, K. Cylindrical molecular brushes: synthesis, characterization, and properties. Prog. Polym. Sci. 33, 759–785 (2008).
Schappacher, M. & Deffieux, A. Imaging of catenated, figure-of-eight, and trefoil knot polymer rings. Angew. Chem. Int. Ed. 48, 5930–5933 (2009).
Schappacher, M. & Deffieux, A. Synthesis of macrocyclic copolymer brushes and their self-assembly into supramolecular tubes. Science 319, 1512–1515 (2008).
Sheiko, S. S. et al. Adsorption-induced scission of carbon-carbon bonds. Nature 440, 191–194 (2006).
Rief, M., Oesterhelt, F., Heymann, B. & Gaub, H. E. Single molecule force spectroscopy on polysaccharides by atomic force microscopy. Science 275, 1295–1297 (1997).
Shi, W. Q. et al. Closed mechanoelectrochemical cycles of individual single-chain macromolecular motors by AFM. Angew. Chem. Int. Ed. 46, 8400–8404 (2007).
Barner, J., Al-Hellani, R., Schluter, A. D. & Rabe, J. P. Synthesis with single macromolecules: covalent connection between a neutral dendronized polymer and polyelectrolyte chains as well as graphene edges. Macromol. Rapid Commun. 31, 362–367 (2010).
Barbara, P. F., Gesquiere, A. J., Park, S.-J. & Lee, Y. J. Single-molecule spectroscopy of conjugated polymers. Acc. Chem. Res. 38, 602–610 (2005).
Lafferentz, L. et al. Conductance of a single conjugated polymer as a continuous function of its length. Science 323, 1193–1197 (2009).
Okawa, Y. et al. Chemical wiring and soldering toward all-molecule electronic circuitry. J. Am. Chem. Soc. 133, 8227–8233 (2011).
Sykora, M., Maxwell, K. A., DeSimone, J. M. & Meyer, T. J. Mimicking the antenna-electron transfer properties of photosynthesis. Proc. Natl Acad. Sci. USA 97, 7687–7691 (2000).
Shimakoshi, H. et al. Photocatalytic function of a polymer-supported B-12 complex with a ruthenium trisbipyridine photosensitizer. Chem. Commun. 47, 6548–6550 (2011).
Alstrum-Acevedo, J. H., Brennaman, M. K. & Meyer, T. J. Chemical approaches to artificial photosynthesis. 2. Inorg. Chem. 44, 6802–6827 (2005).
Dickerson, T. J., Reed, N. N. & Janda, K. D. Soluble polymers as scaffolds for recoverable catalysts and reagents. Chem. Rev. 102, 3325–3344 (2002).
Acknowledgements
J.-F.L. thanks the CNRS, the University of Strasbourg, the International Center for Frontier Research in Chemistry (FRC, Strasbourg) and the European Research Council (Project SEQUENCES – ERC grant agreement no. 258593) for financial support. M.S. and M.O. thank the Ministry of Education, Culture, Sports, Science and Technology, Japan, for financial support through a Grant-in-Aid for Creative Science Research (18GS0209).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ouchi, M., Badi, N., Lutz, JF. et al. Single-chain technology using discrete synthetic macromolecules. Nature Chem 3, 917–924 (2011). https://doi.org/10.1038/nchem.1175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1175
This article is cited by
-
Concurrent control over sequence and dispersity in multiblock copolymers
Nature Chemistry (2022)
-
Scalable preparation and direct visualization of cyclic polymers via self-folding cyclization technique
Science China Chemistry (2022)
-
Regio- and sequence-controlled conjugated topological oligomers and polymers via boronate-tag assisted solution-phase strategy
Nature Communications (2021)
-
High-density information storage in an absolutely defined aperiodic sequence of monodisperse copolyester
Nature Communications (2020)
-
Programmed folding into spiro-multicyclic polymer topologies from linear and star-shaped chains
Communications Chemistry (2020)