Abstract
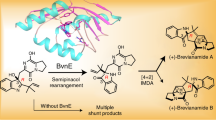
The Diels–Alder reaction is one of the most well-studied, synthetically useful organic transformations. Although it has been postulated that a significant number of naturally occurring substances arise by biosynthetic Diels–Alder reactions, rigorous confirmation of a mechanistically distinct natural Diels–Alderase enzyme remains elusive. Within this context, several related fungi within the Aspergillus genus produce a number of metabolites of opposite absolute configuration, including (+)- or (−)-versicolamide B. These alkaloids are hypothesized to arise via biosynthetic Diels–Alder reactions, implying that each Aspergillus species possesses enantiomerically distinct Diels–Alderases. In this paper, experimental validation of these biosynthetic proposals via deployment of the intramolecular hetero-Diels–Alder reaction as a key step in the asymmetric total syntheses of (+)- and (−)-versicolamide B is described. Laboratory validation of the proposed biosynthetic Diels–Alder construction, coupled with the secondary metabolite profile of the producing fungi, reveals that each Aspergillus species has evolved enantiomerically distinct indole oxidases, as well as enantiomerically distinct Diels–Alderases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
06 April 2009
In the version of this Article originally published, the stereochemical descriptors given in the Methods section for compounds 23, 25, 27 and 29 were incorrect, and the graphical abstract on the Table of Contents page was missing a double bond in (+)-versicolamide B. These errors have now been corrected in the HTML and PDF versions.
References
Croteau, R. Biosynthesis and catabolism of monoterpenoids. Chem. Rev. 87, 929–954 (1987).
Tsuda, M., Kawasaki, N. & Kobayashi, J. Iricinols A and B, first antipodes of manzamine-related alkaloids from an Okinawan marine sponge. Tetrahedron 50, 7957–7960 (1994).
Baldwin, J. E. & Whitehead, R. C. On the biosynthesis of manzamines. Tetrahedron Lett. 33, 2059–2062 (1992).
Williams, R. M., Stocking, E. M. & Sanz-Cevera, J.F. Biosynthesis of prenylated alkaloids derived from tryptophan. Top. Curr. Chem. 209, 97–173 (2000).
Williams, R. M. Total synthesis and biosynthesis of the paraherquamides: an intriguing story of the biological Diels–Alder construction. Chem. Pharm. Bull. 50, 711–740 (2002).
Williams, R. M., Stocking, E. M. & Sanz-Cevera, J. F. Biosynthesis of prenylated alkaloids derived from tryptophan. Top. Curr. Chem. 209, 97–173 (2000).
Qian-Cutrone, J. et al. Stephacidin A and B: two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP Cells. J. Am. Chem. Soc. 124, 14556–14557 (2002).
Martinez-Luis, S. et al. Malbrancheamide, a new calmodulin inhibitor from the fungus Malbranchea aurantiaca. Tetrahedron 62, 1817–1822 (2006).
Porter, A. E. A. & Sammes, P. G. A. Diels–Alder reaction of possible biosynthetic importance. J. Chem. Soc. Chem. Commun. 1103–1104 (1970).
Baldas, J., Birch, A. J. & Russell, R. A. Studies in relation to biosynthesis. Part XLVI. Incorporation of cyclo-l-tryptophyl-l-proline into brevianamide A. J. Chem. Soc. Perkin Trans. I 50–52 (1974).
Greshock, T. J. & Williams, R. W. Improved biomimetic total synthesis of D,L-stephacidin A. Org. Lett. 9, 4255–4258 (2007).
Greshock, T. J., Grubbs, A. W, Tsukamoto, S. & Williams, R. W. A Concise, biomimetic total synthesis of stephacidin A and notoamide B. Angew. Chem. Int. Ed. 46, 2262–2265 (2007).
Williams, R. M., Sanz-Cervera, J. F., Sancenón, F., Marco, J. A. & Halligan, K. Biomimetic Diels–Alder cyclizations for the construction of the brevianamide, paraherquamide, sclerotamide, and VM55599 ring systems. J. Am. Chem. Soc. 120, 1090–1091 (1998).
Williams, R. M., Sanz-Cervera, J. F., Sancenón, F., Marco, J. A. & Halligan, K. Biomimetic Diels–Alder cyclizations for the construction of the brevianamide, paraherquamide, sclerotamide, asperparaline and VM55599 ring systems. Bioorg. Med. Chem. 6, 1233–1241 (1998).
Sanz-Cervera, J. F. et al. A synthetic model for the [4 + 2] cycloaddition in the biosynthesis of the brevianamides, paraherquamides, and related compounds. Tetrahedron 56, 6345–6358 (2000).
Greshock, T. J., Grubbs, A. W. & Williams, R. M. Concise, biomimetic total synthesis of d,l-marcfortine C. Tetrahedron 63, 6124–6130 (2007).
Stocking, E. M., Sanz-Cervera, J. F. & Williams, R. M. Total synthesis of VM55599. Utilization of an intramolecular Diels–Alder cycloaddition of potential biogenetic relevance. J. Am. Chem. Soc. 122, 1675–1683 (2000).
Sanz-Cervera, J. F. & Williams, R. M. Asymmetric total synthesis of (−)-VM55599: establishment of the absolute stereochemistry and biogenetic implications. J. Am. Chem. Soc. 124, 2556–2559 (2002).
Miller, K. A. et al. Biomimetic total synthesis of malbrancheamide and malbrancheamide B. J. Org. Chem. 73, 3116–3119 (2008).
Greshock, T. J. et al. Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew. Chem. Int. Ed. 47, 3573–3577 (2008).
Stocking, E. M. & Williams, R. M. Chemistry and biology of biosynthetic Diels–Alder reactions. Angew. Chem. Int. Ed. 42, 3078–3115 (2003).
Fusetani, N., Asai, N., Matsunaga, S., Honda, K. & Yasumuro, K. Cyclostellettamines A–F, pyridine alkaloids which inhibit binding of methyl quinuclidinyl benzilate (QNB) to muscarinic acetylcholine receptors, from the marine sponge, Stefletta maxitnal. Tetrahedron Lett. 35, 3967–3970 (1994).
Baldwin, J. E. et al. Studies on the biomimetic synthesis of the manzamine alkaloids. Chem. Eur. J. 5, 3154–3161 (1999).
Oikawa, H., Suzuki, Y., Naya, A., Katayama, K. & Ichihara, A. First direct evidence in biological Diels–Alder reaction of incorporation of diene–dienophile precursors in the biosynthesis of solanapyrones. J. Am. Chem. Soc. 116, 3605–3606 (1994).
Ruch, C., Guimarães, W., Udier-Blagovic, M. & Jorgensen, W. L. Macrophomate synthase: QM/MM simulations address the Diels–Alder versus Michael–aldol reaction mechanism. J. Am. Chem. Soc. 127, 3577–3588 (2005).
Domingo, L. R., Sanz-Cervera, J. F., Williams, R. M., Picher, M. T. & Marco, J. A. Biosynthesis of the brevianamides. An ab initio study of the biosynthetic intramolecular Diels–Alder cycloaddition. J. Org. Chem. 62, 1662–1667 (1997).
Domingo, L. R., Zaragozá, R. J. & Williams, R. M. Studies on the biosynthesis of paraherquamide A and VM99955. A theoretical study of intramolecular Diels–Alder cycloaddition. J. Org. Chem. 68, 2895–2902 (2003).
Takayama, H. et al. Stereochemical studies on the Uncaria alkaloid, 3-oxo-7-hydroxy-3,7-secorhynchophylline: the absolute configuration of 3-hydroxyoxindole derivatives. Tetrahedron 55, 6841–6846 (1999).
Adams, L. A., Gray, C. R. & Williams, R. M. Concise synthesis of the core bicyclo[2.2.2]diazaoctane ring common to asperparaline, paraherquamide, and stephacidin alkaloids. Tetrahedron Lett. 45, 4489–4493 (2004).
Adams, L. A., Valente, M. W. N. & Williams, R. M. A concise synthesis of d,l-brevianamide B via a biomimetically-inspired IMDA construction. Tetrahedron 62, 5195–5200 (2006).
Kato, H. et al. Notoamides A-D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. 46, 2254–2256 (2007).
Acknowledgements
This paper is dedicated to Tohru Fukuyama on the occasion of his 60th birthday. Financial support from the US National Institutes of Health (CA70375) is gratefully acknowledged. We are indebted to Alan J. Kennan for measurements of CD spectra and Scott Newkirk for HPLC assistance. We thank Frank R. Stermitz for helpful discussions.
Author information
Authors and Affiliations
Contributions
R.M.W and K.A.M conceived the experiments. K.A.M performed the laboratory experiments and analysed the results. S.T. discovered (−)-versicolamide B as a natural metabolite of a marine-derived Aspergillus species. K.A.M and R.M.W wrote the paper.
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 1295 kb)
Rights and permissions
About this article
Cite this article
Miller, K., Tsukamoto, S. & Williams, R. Asymmetric total syntheses of (+)- and (−)-versicolamide B and biosynthetic implications. Nature Chem 1, 63–68 (2009). https://doi.org/10.1038/nchem.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.110
This article is cited by
-
Vinylogous Michael addition of nitroalkylideneoxindoles to isatylidene-malononitriles in the regio- and diastereoselective synthesis of dispirocyclopentylbisoxindoles
Journal of Chemical Sciences (2023)
-
Electrooxidation enables highly regioselective dearomative annulation of indole and benzofuran derivatives
Nature Communications (2020)
-
Erratum: Asymmetric total syntheses of (+)- and (−)-versicolamide B and biosynthetic implications
Nature Chemistry (2009)