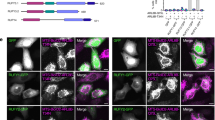
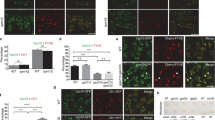
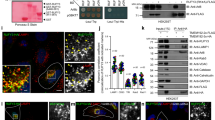
Abstract
The small GTPase Rab6a is involved in the regulation of membrane traffic from the Golgi apparatus towards the endoplasmic reticulum (ER) in a coat complex coatomer protein I (COPI)-independent pathway1,2,3,4,5,6. Here, we used a yeast two-hybrid approach to identify binding partners of Rab6a. In particular, we identified the dynein–dynactin-binding protein Bicaudal-D1 (BICD1), one of the two mammalian homologues of Drosophila Bicaudal-D7,8,9,10. BICD1 and BICD2 colocalize with Rab6a on the trans-Golgi network (TGN) and on cytoplasmic vesicles, and associate with Golgi membranes in a Rab6-dependent manner. Overexpression of BICD1 enhances the recruitment of dynein–dynactin to Rab6a-containing vesicles. Conversely, overexpression of the carboxy-terminal domain of BICD, which can interact with Rab6a but not with cytoplasmic dynein, inhibits microtubule minus-end-directed movement of green fluorescent protein (GFP)–Rab6a vesicles and induces an accumulation of Rab6a and COPI-independent ER cargo in peripheral structures. These data suggest that coordinated action between Rab6a, BICD and the dynein–dynactin complex controls COPI-independent Golgi–ER transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rothman, J. E. & Wieland, F. T. Science 272, 227–234 (1996).
Schekman, R. & Orci, L. Science 271, 1526–1533 (1996).
Girod, A. et al. Nature Cell Biol. 1, 423–430 (1999).
White, J. et al. J. Cell Biol. 147, 743–760 (1999).
Storrie, B., Pepperkok, R. & Nilsson, T. Trends Cell Biol. 10, 385–391 (2000).
Martinez, O. et al. Proc. Natl Acad. Sci. USA 94, 1828–1833 (1997).
Bullock, S. L. & Ish-Horowicz, D. Nature 414, 611–616 (2001).
Hoogenraad, C. C. et al. EMBO J. 20, 4041–4054 (2001).
Baens, M. & Marynen, P. Genomics 45, 601–606 (1997).
Swan, A., Nguyen, T. & Suter, B. Nature Cell Biol. 1, 444–449 (1999).
Johannes, L. & Goud, B. Trends Cell Biol. 8, 158–162 (1998).
Zerial, M. & McBride, H. Nature Rev. Mol. Cell Biol. 2, 107–117 (2001).
Pfeffer, S. R. Trends Cell Biol. 11, 487–491 (2001).
Gross, S. P., Welte, M. A., Block, S. M. & Wieschaus, E. F. J. Cell Biol. 156, 715–724 (2002).
Echard, A. et al. Science 279, 580–585 (1998).
King, S. J. & Schroer, T. A. Nature Cell Biol. 2, 20–24 (2000).
Martinez, O. et al. J. Cell Biol. 127, 1575–1588 (1994).
Hoogenraad, C. C., Akhmanova, A., Grosveld, F., De Zeeuw, C. I. & Galjart, N. J. Cell Sci. 113, 2285–2297 (2000).
Schiedel, A. C., Barnekow, A. & Mayer, T. FEBS Lett. 376, 113–119 (1995).
Johannes, L., Tenza, D., Antony, C. & Goud, B. J. Biol. Chem. 272, 19554–19561 (1997).
Elbashir, S. M. et al. Nature 411, 494–498 (2001).
Akhmanova, A. et al. Cell 104, 923–935 (2001).
Suter, B., Romberg, L. M. & Steward, R. Genes Dev. 3, 1957–1968 (1989).
Purcell, K. & Artavanis-Tsakonas, S. J. Cell Biol. 146, 731–740 (1999).
Acknowledgements
We thank I. G. Macara, S. R. Pfeffer, D. Gallwitz, E. G. Berger and H. P. Hauri for providing reagents. We also thank M. Rosing, K. Bilbilis, M. Koester, E. Ossendorf and A. Theil for experimental assistance. This research was supported by grants from the Netherlands Organisation for Scientific Research (ZonMw/900-00-001), the Erasmus University and grants from Fonds der Chemischen Industrie (FCI) and Deutsche Forschungsgemeinschaft (DFG) to A.B. This study contains major parts of the PhD thesis of T.M. T.M. is a fellow of the Graduiertenfoerderung of Nordrhein-Westfalen.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information: Additional Materials and methods
Supplementary information: Tables (DOC 63 kb)
Figure S1
Specificity of the #2293 and #2296 antibodies using immunofluorescence microscopy and colocalisation between BICD proteins and Golgi markers (JPG 239 kb)
Figure S2
Silencing of Rab6 in HeLa cells. (JPG 1160 kb)
Figure S3
BICD2-C causes peripheral accumulation of COPI-independent Golgi-ER cargo, but has no effect on GM130 and γ-adaptin (JPG 1126 kb)
Figure S4
BICD2-C has no effect on the microtubule network and induces no accumulation of dynein or dynactin. (JPG 713 kb)
Movie 1
GFP-Rab6A movement in HeLa cells (AVI 903 kb)
Movie 2
GFP-Rab6A movement in HeLa cells expressing BICD2-C (AVI 796 kb)
Rights and permissions
About this article
Cite this article
Matanis, T., Akhmanova, A., Wulf, P. et al. Bicaudal-D regulates COPI-independent Golgi–ER transport by recruiting the dynein–dynactin motor complex. Nat Cell Biol 4, 986–992 (2002). https://doi.org/10.1038/ncb891
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb891
This article is cited by
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Subcellular spatial transcriptomics identifies three mechanistically different classes of localizing RNAs
Nature Communications (2022)
-
Recycling of autophagosomal components from autolysosomes by the recycler complex
Nature Cell Biology (2022)
-
Loss of BICD2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy
Acta Neuropathologica Communications (2020)
-
O-cyclic phytosphingosine-1-phosphate stimulates HIF1α-dependent glycolytic reprogramming to enhance the therapeutic potential of mesenchymal stem cells
Cell Death & Disease (2019)