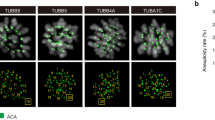
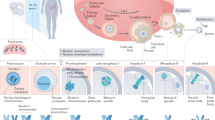
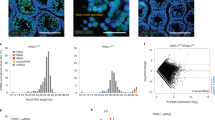
Abstract
Successful reproduction in mammals requires a competent egg, which is formed during meiosis through two assymetrical cell divisions. Here, we show that a recently identified formin homology (FH) gene, formin-2 (Fmn2), is a maternal-effect gene that is expressed in oocytes and is required for progression through metaphase of meiosis I. Fmn2−/− oocytes cannot correctly position the metaphase spindle during meiosis I and form the first polar body. We demonstrate that Fmn2 is required for microtubule-independent chromatin positioning during metaphase I. Fertilization of Fmn2−/− oocytes results in polyploid embryo formation, recurrent pregnancy loss and sub-fertility in Fmn2−/− females. Injection of Fmn2 mRNA into Fmn2-deficient oocytes rescues the metaphase I block. Given that errors in meiotic maturation result in severe birth defects and are the most common cause of chromosomal aneuploidy and pregnancy loss in humans, studies of Fmn2 may provide a better understanding of infertility and birth defects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Matova, N. & Cooley, L. Comparative aspects of animal oogenesis. Dev. Biol. 231, 291–320 (2001).
Elvin, J. A. & Matzuk, M. M. Mouse models of ovarian failure. Rev. Reprod. 3, 183–195 (1998).
Albertini, D. F. & Carabatsos, M. J. Comparative aspects of meiotic cell cycle control in mammals. J. Mol. Med. 76, 795–799 (1998).
Wassarman, P. M. & Albertini, D. F. The mammalian ovum in The Physiology of Reproduction, 2nd edition (eds Knobil, E. & Neill, J. D.) 79–122 (Raven Press, New York, New York, 1994).
Gardner, R. L. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal–vegetal axis of the zygote in the mouse. Development 124, 289–301 (1997).
Brunet, S. et al. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 146, 1–12 (1999).
Schatten, H., Schatten, G., Mazia, D., Balczon, R. & Simerly, C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl Acad. Sci. USA 83, 105–109 (1986).
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev. Genet. 2, 280–291 (2001).
Verlhac, M. H. et al. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 122, 815–822 (1996).
Pittman, D. L. et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1, 697–705. (1998).
Edelmann, W. et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell 85, 1125–1134 (1996).
Zeller, R. et al. Formin defines a large family of morphoregulatory genes and functions in establishment of the polarising region. Cell Tissue Res. 296, 85–93 (1999).
Emmons, S. et al. Cappuccino, a Drosophila maternal effect gene required for polarity of the egg and embryo, is related to the vertebrate limb deformity locus. Genes Dev. 9, 2482–2494 (1995).
Castrillon, D. H. & Wasserman, S. A. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120, 3367–3377 (1994).
Leader, B. & Leder, P. Formin-2, a novel formin homology protein of the cappuccino subfamily, is highly expressed in the developing and adult central nervous system. Mech. Dev. 93, 221–231 (2000).
Bedford, M. T., Chan, D. C. & Leder, P. FBP WW domains and the Abl SH3 domain bind to a specific class of proline-rich ligands. EMBO J. 16, 2376–2383 (1997).
Tominaga, T. et al. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell 5, 13–25 (2000).
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nature Cell Biol. 1, 136–143 (1999).
Pruyne, D. et al. Role of formins in actin assembly: nucleation and barbed end association. Science 297, 612–615 (2002).
Maro, B. et al. Cytoskeleton organization during oogenesis, fertilization, and preimplantation development of the mouse. Int. J. Dev. Biol. 34, 127–137 (1990).
Maro, B., Johnson, M. H., Webb, M. & Flach, G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J. Embryol. Exp. Morphol. 92, 11–32 (1986).
Longo, F. J. & Chen, D. Y. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev. Biol. 107, 382–394 (1985).
Kaufman, M. H. & Speirs, S. The postimplantation development of spontaneous digynic triploid embryos in LT/Sv strain mice. Development 101, 383–391 (1987).
Eppig, J. J., Schultz, R. M., O'Brien, M. & Chesnel, F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev. Biol. 164, 1–9 (1994).
Schatten, G., Simerly, C. & Schatten, H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule- mediated motility during mammalian fertilization. Proc. Natl Acad. Sci. USA 82, 4152–4156 (1985).
Zernicka-Goetz, M. Fertile offspring derived from mammalian eggs lacking either animal or vegetal poles. Development 125, 4803–4808 (1998).
Weber, R. J., Pedersen, R. A., Wianny, F., Evans, M. J. & Zernicka-Goetz, M. Polarity of the mouse embryo is anticipated before implantation. Development 126, 5591–5598 (1999).
Lee, L., Klee, S. K., Evangelista, M., Boone, C. & Pellman, D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947–961 (1999).
Kato, T. et al. Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J. Cell Sci. 114, 775–784 (2001).
Heil-Chapdelaine, R. A., Adames, N. R. & Cooper, J. A. Formin' the connection between microtubules and the cell cortex. J. Cell Biol. 144, 809–811 (1999).
Evangelista, M., Pruyne, D., Amberg, D. C., Boone, C. & Bretscher, A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nature Cell Biol. 4, 32–41 (2002).
Sawin, K. E. Cell polarity: following formin function. Curr. Biol. 12, R6–R8 (2002).
Yarm, F., Sagot, I. & Pellman, D. The social life of actin and microtubules: interaction versus cooperation. Curr. Opin. Microbiol. 4, 696–702 (2001).
Jackson, S. M. & Berg, C. A. Soma-to-germline interactions during Drosophila oogenesis are influenced by dose-sensitive interactions between cut and the genes cappuccino, ovarian tumor and agnostic. Genetics 153, 289–303 (1999).
Magie, C. R., Meyer, M. R., Gorsuch, M. S. & Parkhurst, S. M. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development 126, 5353–5364 (1999).
Afshar, K., Stuart, B. & Wasserman, S. A. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 127, 1887–1897 (2000).
Lynch, E. D. et al. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science 278, 1315–1318 (1997).
Hill, J. A. & Choi, B. C. Maternal immunological aspects of pregnancy success and failure. J. Reprod. Fertil. (Suppl.) 55, 91–97 (2000).
Weiss, R. S., Enoch, T. & Leder, P. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 14, 1886–1898 (2000).
Deng, C., Wynshaw-Boris, A., Zhou, F., Kuo, A. & Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84, 911–921 (1996).
Hogan, B., Beddington, R., Constantini, F. & Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual 185–186 (Cold Spring Harbor Press, 1994).
Das, S. K. et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120, 1071–1083 (1994).
de Vantery, C., Stutz, A., Vassalli, J. D. & Schorderet-Slatkine, S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev. Biol. 187, 43–54 (1997).
Carabatsos, M. J., Combelles, C. M., Messinger, S. M. & Albertini, D. F. Sorting and reorganization of centrosomes during oocyte maturation in the mouse. Microsc. Res. Tech. 49, 435–444 (2000).
Acknowledgements
We are indebted to C. O'Hara and M. Michelman for their assistance in creating and maintaining the targeted embryonic cell lines required for deletion of the Fmn2 gene. We thank D. Albertini for his advice and insight about oocyte development and critical review of this manuscript. We thank J. Eppig and M. Kaufman for advice regarding methods for blastocyst karyotyping. We are grateful to J. Seidman, C. Tabin, R. Weiss, N. Benvenisty, C. Racowsky, D. Schust, V. Makarov and H. Chen for their advice about this manuscript. B.L. was supported by a Howard Hughes Medical Institute (HHMI) predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures and table
Figure S1. RNA blot analysis of Fmn2 mRNA expression in Fmn2-targeted mice. (PDF 315 kb)
Figure S2. Immunoprecipitations of the Fmn2 protein in brain and ovary.
Figure S3. In vivo translation of Fmn2 expression constructs.
Table SI Litter size in Fmn2+/+, Fmn2+/-, and Fmn2-/- female mice.
Rights and permissions
About this article
Cite this article
Leader, B., Lim, H., Carabatsos, M. et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol 4, 921–928 (2002). https://doi.org/10.1038/ncb880
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb880
This article is cited by
-
Heterozygous FMN2 missense variant found in a family case of premature ovarian insufficiency
Journal of Ovarian Research (2022)
-
Lima1 mediates the pluripotency control of membrane dynamics and cellular metabolism
Nature Communications (2022)
-
Cytoplasmic forces functionally reorganize nuclear condensates in oocytes
Nature Communications (2022)
-
Achiasmatic meiosis in the unisexual Amazon molly, Poecilia formosa
Chromosome Research (2022)
-
Maternal effect factors that contribute to oocytes developmental competence: an update
Journal of Assisted Reproduction and Genetics (2022)