Abstract
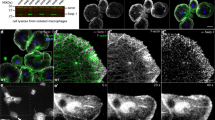
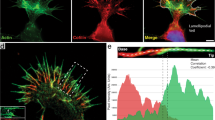
Ephrins and Eph receptors are involved in axon guidance and cellular morphogenesis. An interaction between ephrin and Eph receptors elicits neuronal growth-cone collapse through cytoskeletal disassembly. When NIH3T3 cells were plated onto an ephrinA1-coated surface, the cells both adhered and spread. Adhesion and spreading proceeded concomitantly with changes in both the actin and microtubule cytoskeleton. EphA2, focal adhesion kinase (FAK) and p130cas were identified as the major ephrin-dependent phosphotyrosyl proteins during the ephrin-induced morphological changes. Mouse embryonic fibroblasts (MEFs) derived from FAK−/− and p130cas−/− mice had severe defects in ephrinA1-induced cell spreading, which were reversed after re-expression of FAK or p130cas, respectively. Expression of a constitutively active EphA2 induced NIH3T3 cells to undergo identical, but ligand-independent, morphological changes. These data show that ephrinA1 can induce cell adhesion and actin cytoskeletal changes in fibroblasts in a FAK- and p130cas-dependent manner, through activation of the EphA2 receptor. The finding that ephrin–Eph signalling can result in actin cytoskeletal assembly, rather than disassembly, has many implications for ephrin–Eph responses in other cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Pasquale, E. B. The Eph family of receptors. Curr. Opin. Cell Biol. 9, 608–615 (1997).
Flanagan, J. G. & Vanderhaeghen, P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345 (1998).
Holland, S. J. et al. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722–725 (1996).
Bruckner, K., Pasquale, E. B. & Klein, R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275, 1640–1643 (1997).
Monschau, B. et al. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. EMBO J. 16, 1258–1267 (1997).
Pasquale, E. B. & Klein, R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275, 1640–1643 (1997).
Wilkinson, D. G. Multiple roles of EPH receptors and ephrins in neural development. Nature Rev. Neurosci. 2, 155–164 (2001).
Gerlai, R. Eph receptors and neural plasticity. Nature Rev. Neurosci. 2, 205–209 (2001).
Klein, R. Excitory Eph receptors and adhesive ephrin ligands. Curr. Opin. Cell Biol. 13, 196–203 (2001).
Davy, A. et al. Compartmentalised signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 13, 3125–3135 (1999)
Davy, A. & Robbins, S. M. Ephrin-A5 modulates cell adhesion and morphology in an integrin dependent manner. EMBO J. 19, 5396–5405 (2001).
Lu, Q., Sun, E. E., Klein, R. S. & Flanagan, J. G. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105, 69–79 (2001).
Gao, P. P., Sun, C. H., Zhou, X. F., DiCicco-Bloom, E. & Zhou, R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J. Neurosci. Res. 60, 427–436 (2000).
Holmberg, J., Clarke, D.L. & Frisen, J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408, 203–206 (2000).
Schlaepfer, D. D., Hauck, C. R. & Sieg, D. J. Signalling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478 (1999).
Parsons, J. T., Martin, K. H., Slack, J. K., Taylor, J. M. & Weed. S. A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606–5613 (2000).
Hauck, C. R., Klingbeil, C. K. & Schlaepfer, D. D. Focal adhesion kinase functions as a receptor-proximal signaling component required for directed cell migration. Immunol. Res. 21, 293–303 (2000).
Polte, T. R. & Hanks, S. K. Interaction between focal adhesion kinase and crk-associated tyrosine kinase substrate p130cas. Proc. Natl Acad. Sci. USA 92, 10678–10682 (1995).
Harte, M. T., Macklem, M., Weidow, C. L., Parsons, J. T. & Bouton, A. H. Identification of two focal adhesion targeting sequences in the adapter molecule p130(Cas). Biochim. Biophys. Acta 1499, 34–48 (2000).
Sieg, D. J., Hauck, C. R. & Schlaepfer, D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112, 2677–2691 (1999).
Huynh-Do, U. et al. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through α5β3 and α5β1 integrins. EMBO J. 18, 2165–2173 (1999).
Miao, H., Burnett, E., Kinch, M., Simon, E. & Wang, B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nature Cell Biol. 2, 62–69 (2000).
Gu, C. & Park, S. The EphA8 receptor regulates integrin activity through p110γ phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell. Biol. 14, 4579–4597 (2001).
Becker, E. et al. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol. Cell. Biol. 5, 1537–1545 (2000).
Huai, J. & Drescher, U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem. 276, 6689–6694 (2001).
Zhang, Z., Vuori, K., Wang, H., Reed, J. C. & Ruoslahti, E. Integrin activation by R-ras. Cell 85, 61–69 (1996).
Berrier, A. L., Mastrangelo, A. M., Downward, J., Ginsberg, M. & LaFlamme, S. E. Activated R-ras, Rac1, PI 3-kinase and PKCγ can each restore cell spreading inhibited by isolated integrin β1 cytoplasmic domains. J. Cell Biol. 151, 1549–1560 (2000).
Zou, J. X. et al. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl Acad. Sci. USA 96, 13813–13818 (1999).
Dodelet, V. C., Pazzagli, C., Zisch, A. H, Hauser, C. A. & Pasquale, E. B. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J. Biol. Chem. 274, 31941–31946 (1999).
Miao, H. et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nature Cell Biol. 3, 527–530 (2001).
Schlaepfer, D. D., Hanks, S. K., Hunter, T. & van der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372, 786–791 (1994).
Schlaepfer, D. D. & Hunter, T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 16, 5623–5633 (1996).
Cary, L. A., Han, D. C., Polte, T. R., Hanks, S. K. & Guan, J. L. Identification of p130cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140, 211–221 (1998)
Klingbeil, C. K. et al. Targetting Pyk2 to β1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J. Cell Biol. 152, 97–110 (2001).
Ruest, P. J., Roy, S., Shi, E., Mernaugh, R. L. & Hanks, S. K. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 11, 41–48 (2000).
Ilic, D. et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 (1995).
Honda, H. et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130cas. Nature Genet. 19, 361–365 (1998).
Honda, H., Nakamoto, T., Sakai, R. & Hirai, H. p130cas, an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem. Biophys. Res. Commun. 262, 25–30 (1999).
Gonzatti-Haces, M. et al. Characterisation of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc. Natl Acad. Sci. USA 85, 21–25 (1988).
Rodrigues, G. A. & Park, M. Dimerisation mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol. Cell. Biol. 13, 6711–6722 (1993).
Liu, A. Y. Differential expression of cell surface molecules in prostate cancer cells. Cancer Res. 60, 3429–3434 (2000).
Torring, N., Jorgensen, P. E., Sorensen, B. S. & Nexo, E. Increased expression of heparin binding EGF (HB-EGF), amphiregulin, TGFα and epiregulin in androgen-independent prostate cancer cell lines. Anticancer Res. 20, 91–95 (2000).
Stanzione, R. et al. Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab. Invest. 81, 51–59 (2001).
Walker-Daniels, J. et al. Overexpression of the EphA2 Tyrosine Kinase in Prostate Cancer. Prostate 41, 275–280 (1999).
Wahl, S., Barth, H., Ciossek, T., Aktories, K. & Mueller, B. K. Ephrin-A5 Induces Collapse of Growth Cones by Activating Rho and Rho Kinase. J. Cell Biol. 149, 263–270 (2000).
Zhou, F-Q. & Cohan, C. S. Growth Cone Collapse through Coincident loss of Actin Bundles and Leading Edge Actin without Actin Depolymerisation. J. Cell Biol. 153, 1071–1083 (2001).
Holland, S. J. et al. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 16, 3877–3888 (1997).
Zisch, A. H., Kalo, M. S., Chong, L. D. & Pasquale, E. B. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16, 2657–2670 (1998).
Yu, H. H., Zisch, A. H., Dodelet, V. C. & Pasquale, E. B. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 20, 3995–4006 (2001).
Elowe, S. Holland, S. J., Kulkarni, S. & Pawson, T. Downregulation of the ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol. Cell. Biol. 21, 7429–7441 (2001).
Schmucker, D. & Zipursky, S. L. Signalling downstream of Eph receptors and ephrin ligands. Cell 105, 701–704 (2001).
Shamah, S. M. et al. Epha receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 (2001).
Lindberg, R. A. & Hunter, T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol. Cell. Biol. 10, 6316–6324 (1990).
Jiang, W. et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell 2, 877–885 (1998).
Acknowledgements
Funding for parts of this work was provided by a Human Frontiers Science Program Organisation long-term fellowship and an Amgen Inc. fellowship (to N.C.) as well as National Institutes of Health grants from the National Cancer Institute, CA141915, CA39780 and CA82863 (to T.H.). We are indebted to J. Meisenhelder, S. Simon, H. Mondala and M. Rosenthal for considerable help and expert technical assistance. We also thank C. Vaziri for numerous helpful discussions and R. Lindberg, J. Ruiz, L. Robertson, D. Schlaepfer, N. Somia, W. Jiang and B. Seed for generously donating their time, reagents and expertise. We are especially grateful to R.Gage, B. Summers and L. Kitabayashi for access to and help with confocal microscopy, and J. Simon for invaluable help with Adobe Photoshop. T.H. is a Frank and Elise Schilling American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Figure S1. Plating NIH3T3 cells on to ephrinA1 induces cell adhesion and spreading in a large percentage of cells and this is mimicked by expression of an activated EphA2 receptor. (PDF 228 kb)
Figure S2. Profiling ephrinA1-responsive EphA receptors in NIH3T3 cells. Serum starved NIH3T3 cells were treated with 2µg/ml ephrinA1-Fc or buffer control for 30 minutes.
Figure S3. Adherence of NIH3T3 and PC3 cells to culture dishes coated with differing concentrations of EphrinA1.
Rights and permissions
About this article
Cite this article
Carter, N., Nakamoto, T., Hirai, H. et al. EphrinA1-induced cytoskeletal re-organization requires FAK and p130cas. Nat Cell Biol 4, 565–573 (2002). https://doi.org/10.1038/ncb823
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb823
This article is cited by
-
Dysregulated erythropoietin-producing hepatocellular receptor A2 (EphA2) is involved in tubal pregnancy via regulating cell adhesion of the Fallopian tube epithelial cells
Reproductive Biology and Endocrinology (2018)
-
Functional non-coding polymorphism in an EPHA2 promoter PAX2 binding site modifies expression and alters the MAPK and AKT pathways
Scientific Reports (2017)
-
EphrinB2/EphB4 pathway in postnatal angiogenesis: a potential therapeutic target for ischemic cardiovascular disease
Angiogenesis (2016)
-
Cell segregation in the vertebrate hindbrain: a matter of boundaries
Cellular and Molecular Life Sciences (2015)
-
Therapeutic targeting of EPH receptors and their ligands
Nature Reviews Drug Discovery (2014)