Abstract
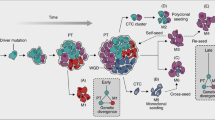
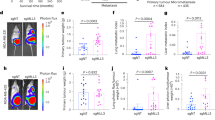
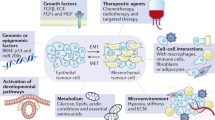
Metastasis is a multistep process that involves local tumour invasion followed by dissemination to, and re-establishment at, distant sites. Here we show that during multistage tumorigenesis, discrete expression thresholds of activated Smad2 and H-ras are sequentially surpassed, driving tumour progression through distinct phases from a differentiated squamous carcinoma to a motile invasive stage, followed by an overt change from epithelial to mesenchymal cell type, finally culminating in metastatic tumour spread. Smad2 activation alone induces migration of tumour cells. Elevated H-ras levels, however, are required for nuclear accumulation of Smad2, both of which are essential for the epithelial–mesenchymal transition (EMT). Having undergone EMT, fibroblastoid carcinoma cells with elevated levels of activated Smad2, gain the capability to spread to a wide variety of tissues by a further increase in Smad2 expression. These findings have far-reaching implications for the prevention of tumour growth, invasion and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signalling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Akhurst, R. J. & Derynck, R. TGF-β signalling in cancer — a double-edged sword. Trends Cell Biol 11, S44–S51 (2001).
Cui, W. et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 86, 531–542 (1996).
Caulin, C., Scholl, F. G., Frontelo, P., Gamallo, C. & Quintanilla, M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-β 1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth Differ. 6, 1027–1035 (1995).
Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Oft, M. et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477 (1996).
Oft, M., Heider, K. H. & Beug, H. TGFβ signalling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8, 1243–1252 (1998).
Portella, G. et al. TGFβ is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for invasion and metastasis. Cell Growth Differ. 9, 393–404 (1998).
Bhowmick, N. A. et al. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27–36 (2001).
Bakin, A. V., Tomlinson, A. K., Bhowmick, N. A., Moses, H. L. & Arteaga, C. L. Phosphatidylinositol-3 kinase function is required for TGFβ-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 (2000).
Piek, E., Moustakas, A., Kurisaki, A., Heldin, C. H. & ten Dijke, P. TGF-β type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J. Cell Sci. 112, 4557–4568 (1999).
Lehmann, K. et al. Raf induces TGFb production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14, 2610–2622 (2000).
Arsura, M., Mercurio, F., Oliver, A. L., Thorgeirsson, S. S. & Sonenshein, G. E. Role of the IkappaB kinase complex in oncogenic Ras- and Raf-mediated transformation of rat liver epithelial cells. Mol. Cell. Biol. 20, 5381–5391 (2000).
Janda, E. et al. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 (2002).
Kretzschmar, M., Doody, J., Timokhina, I. & Massague, J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 13, 804–816 (1999).
Whitman, M. & Melton, D. A. Involvement of p21ras in Xenopus mesoderm induction. Nature 357, 252–254 (1992).
Umbhauer, M., Marshall, C. J., Mason, C. S., Old, R. W. & Smith, J. C. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature 376, 58–62 (1995).
Green, J. B., New, H. V. & Smith, J. C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell 71, 731–739 (1992).
Baker, J. C. & Harland, R. M. A novel mesoderm inducer, Madr2, functions in the activin signal transduction pathway. Genes Dev. 10, 1880–1889 (1996).
Nomura, M. & Li, E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature 393, 786–790 (1998).
Buchmann, A., Ruggeri, B., Klein-Szanto, A. J. & Balmain, A. Progression of squamous carcinoma cells to spindle carcinomas of mouse skin is associated with an imbalance of H-ras alleles on chromosome 7. Cancer Res. 51, 4097–4101 (1991).
Burns, P. A. et al. Loss of heterozygosity and mutational alterations of the p53 gene in skin tumours of interspecific hybrid mice. Oncogene 6, 2363–2369 (1991).
Abe, M. et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 (1994).
Macias-Silva, M. et al. MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell 87, 1215–1224 (1996).
Massague, J. & Wotton, D. Transcriptional control by the TGF-β/Smad signalling system. EMBO J. 19, 1745–1754 (2000).
Miyazono, K., ten Dijke, P. & Heldin, C. H. TGF-β signaling by Smad proteins. Adv. Immunol. 75, 115–157 (2000).
Seftor, R. E. Role of the b3 integrin subunit in human primary melanoma progression: multifunctional activities associated with α(v)3 integrin expression. Am. J. Pathol. 153, 1347–1351 (1998).
Xie, W. et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 62, 497–505 (2002).
Frame, S. & Balmain, A. Integration of positive and negative growth signals during ras pathway activation in vivo. Curr. Opin. Genet. Dev. 10, 106–113 (2000).
Bos, J. L. ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 (1989).
Derynck, R. et al. Human transforming growth factor-b complementary DNA sequence and expression in normal and transformed cells. Nature 316, 701–705 (1985).
Friedman, E. et al. High levels of transforming growth factor b 1 correlate with disease progression in human colon cancer. Cancer Epidemiol. Biomarkers Prev. 4, 549–554 (1995).
Shim, K. S., Kim, K. H., Han, W. S. & Park, E. B. Elevated serum levels of transforming growth factor-b1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer 85, 554–561 (1999).
Green, J. B. & Smith, J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate Nature 347, 391–394 (1990).
Massague, J. & Chen, Y. G. Controlling TGF-β signaling. Genes Dev. 14, 627–644 (2000).
Acknowledgements
This work was supported by grant K01 CA84244 from the NCI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oft, M., Akhurst, R. & Balmain, A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol 4, 487–494 (2002). https://doi.org/10.1038/ncb807
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb807
This article is cited by
-
LncRNA CRLM1 inhibits apoptosis and promotes metastasis through transcriptional regulation cooperated with hnRNPK in colorectal cancer
Cell & Bioscience (2022)
-
Rab31-dependent regulation of transforming growth factor ß expression in breast cancer cells
Molecular Medicine (2021)
-
TGFβ biology in cancer progression and immunotherapy
Nature Reviews Clinical Oncology (2021)
-
Epigenetic regulation of TGF-β-induced EMT by JMJD3/KDM6B histone H3K27 demethylase
Oncogenesis (2021)
-
TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1
Nature (2020)