Abstract
Competition among RNAs to bind miRNA is proposed to influence biological systems. However, the role of this competition in disease onset is unclear. Here, we report that TYRP1 mRNA, in addition to encoding tyrosinase-related protein 1 (TYRP1), indirectly promotes cell proliferation by sequestering miR-16 on non-canonical miRNA response elements. Consequently, the sequestered miR-16 is no longer able to repress its mRNA targets, such as RAB17, which is involved in melanoma cell proliferation and tumour growth. Restoration of miR-16 tumour-suppressor function can be achieved in vitro by silencing TYRP1 or increasing miR-16 expression. Importantly, TYRP1-dependent miR-16 sequestration can also be overcome in vivo by using small oligonucleotides that mask miR-16-binding sites on TYRP1 mRNA. Together, our findings assign a pathogenic non-coding function to TYRP1 mRNA and highlight miRNA displacement as a promising targeted therapeutic approach for melanoma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hausser, J. & Zavolan, M. Identification and consequences of miRNA-target interactions—beyond repression of gene expression. Nat. Rev. Genet. 15, 599–612 (2014).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Chi, S. W., Hannon, G. J. & Darnell, R. B. An alternative mode of microRNA target recognition. Nat. Struct. Mol. Biol. 19, 321–327 (2012).
Agarwal, V., Bell, G. W., Nam, J.-W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (2015).
Kim, D. et al. General rules for functional microRNA targeting. Nat. Genet. 48, 1517–1526 (2016).
Loeb, G. B. et al. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 48, 760–770 (2012).
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 (2013).
Khorshid, M., Hausser, J., Zavolan, M. & van Nimwegen, E. A biophysical miRNA–mRNA interaction model infers canonical and noncanonical targets. Nat. Methods 10, 253–255 (2013).
Grosswendt, S. et al. Unambiguous identification of miRNA: target site interactions by different types of ligation reactions. Mol. Cell 54, 1042–1054 (2014).
Ebert, M. S., Neilson, J. R. & Sharp, P. A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 4, 721–726 (2007).
Franco-Zorrilla, J. M. et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39, 1033–1037 (2007).
Giusti, S. A. et al. MicroRNA-9 controls dendritic development by targeting REST. eLife 3, 1–22 (2014).
Reichel, M., Li, Y., Li, J. & Millar, A. A. Inhibiting plant microRNA activity: molecular SPONGEs, target MIMICs and STTMs all display variable efficacies against target microRNAs. Plant Biotechnol. J. 13, 915–926 (2015).
Tay, Y. et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147, 344–357 (2011).
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010).
Karreth, F. A. et al. In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147, 382–395 (2011).
Karreth, F. A. et al. The BRAF pseudogene functions as a competitive endogenous RNA and induces lymphoma in vivo. Cell 161, 319–332 (2015).
Thomson, D. W. & Dinger, M. E. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 17, 272–283 (2016).
Tay, Y., Rinn, J. & Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Guo, J. U., Agarwal, V., Guo, H. & Bartel, D. P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 15, 409 (2014).
Memczak, S., Jens, M., Elefsinioti, A. & Torti, F. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
Zheng, Q. et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 7, 11215 (2016).
Migault, M., Donnou-Fournet, E., Galibert, M.-D. & Gilot, D. Definition and identification of small RNA sponges: focus on miRNA sequestration. Methods 117, 35–47 (2017).
Boissy, R. E., Sakai, C., Zhao, H., Kobayashi, T. & Hearing, V. J. Human tyrosinase related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp. Dermatol. 7, 198–204 (1998).
Ghanem, G. & Journe, F. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol. Oncol. 5, 150–155 (2011).
El Hajj, P. et al. SNPs at miR-155 binding sites of TYRP1 explain discrepancy between mRNA and protein and refine TYRP1 prognostic value in melanoma. Br. J. Cancer 113, 91–98 (2015).
El Hajj, P. et al. Tyrosinase-related protein 1 mRNA expression in lymph node metastases predicts overall survival in high-risk melanoma patients. Br. J. Cancer 108, 1641–1647 (2013).
Journe, F. et al. TYRP1 mRNA expression in melanoma metastases correlates with clinical outcome. Br. J. Cancer 105, 1726–1732 (2011).
Li, J. et al. Evidence for positive selection on a number of microRNA regulatory interactions during recent human evolution. PLoS Genet. 8, 1–12 (2012).
Kittler, R. et al. Genome-scale RNAi profiling of cell division in human tissue culture cells. Nat. Cell Biol. 9, 1401–1412 (2007).
Li, C.-Y. Y. et al. The effect of antisense tyrosinase-related protein 1 on melanocytes and malignant melanoma cells. Br. J. Dermatol. 150, 1081–1090 (2004).
Tominaga, K. et al. Competitive regulation of nucleolin expression by HuR and miR-494. Mol. Cell. Biol. 31, 4219–4231 (2011).
Rehmsmeier, M., Steffen, P., Hochsmann, M. & Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 10, 1507–1517 (2004).
Selbach, M. et al. Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 (2008).
Pekarsky, Y. & Croce, C. M. Role of miR-15/16 in CLL. Cell Death Differ. 22, 6–11 (2014).
Poell, J. B. et al. A functional screen identifies specific microRNAs capable of inhibiting human melanoma cell viability. PLoS ONE 7, e43569 (2012).
Ebert, M. S. & Sharp, P. A. MicroRNA sponges: progress and possibilities. RNA 16, 2043–2050 (2010).
Hartmann, P. et al. Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nat. Commun. 7, 10521 (2016).
Wynendaele, J. et al. An illegitimate microRNA target site within the 3′ UTR of MDM4 affects ovarian cancer progression and chemosensitivity. Cancer Res. 70, 9641–9649 (2010).
Messina, A. et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci. 19, 835–844 (2016).
Leucci, E. et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531, 518–522 (2016).
Liu, Q. et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 36, 5391–5404 (2008).
Kang, W. et al. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol. Cancer 14, 1–10 (2015).
Falkenius, J. et al. High expression of glycolytic and pigment proteins is associated with worse clinical outcome in stage III melanoma. Melanoma Res. 23, 452–460 (2013).
Rose, A. A. N. et al. MAPK pathway inhibitors sensitize BRAF-mutant melanoma to an antibody-drug conjugate targeting GPNMB. Clin. Cancer Res. 22, 6088–6098 (2016).
Grimson, A. et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 (2007).
Garcia, D. M. et al. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat. Struct. Mol. Biol. 18, 1139–1146 (2011).
Arvey, A., Larsson, E., Sander, C., Leslie, C. S. & Marks, D. S. Target mRNA abundance dilutes microRNA and siRNA activity. Mol. Syst. Biol. 6, 363 (2010).
Luna, J. M. et al. Hepatitis C virus RNA functionally sequesters miR-122. Cell 160, 1099–1110 (2015).
Leung, A. K. L. The whereabouts of microRNA actions: cytoplasm and beyond. Trends Cell Biol. 25, 601–610 (2015).
Alonso-Curbelo, D. et al. RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 26, 61–76 (2014).
Vizoso, M. et al. Epigenetic activation of a cryptic TBC1D16 transcript enhances melanoma progression by targeting EGFR. Nat. Med. 21, 741–750 (2015).
Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016).
Verfaillie, A. et al. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nat. Commun. 6, 6683 (2015).
David, S. et al. In vivo imaging of DNA lipid nanocapsules after systemic administration in a melanoma mouse model. Int. J. Pharm. 423, 108–115 (2012).
Bonci, D. et al. The miR-15a–miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 14, 1271–1277 (2008).
Keene, J. D., Komisarow, J. M. & Friedersdorf, M. B. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 1, 302–307 (2006).
Kim, H. H. et al. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 (2009).
Le Brigand, K. & Barbry, P. Mediante: a web-based microarray data manager. Bioinformatics 23, 1304–1306 (2007).
Gilot, D. et al. RNAi-based screening identifies kinases interfering with dioxin-mediated up-regulation of CYP1A1 activity. PLoS ONE 6, e18261 (2011).
Krayem, M. et al. Prominent role of cyclic adenosine monophosphate signalling pathway in the sensitivity of WTBRAF/WTNRAS melanoma cells to vemurafenib. Eur. J. Cancer 50, 1310–1320 (2014).
Smyth, G. K., Michaud, J. & Scott, H. S. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21, 2067–2075 (2005).
Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The SVA package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883 (2012).
Acknowledgements
The authors thank the Gene Expression and Oncogenesis team, especially K. Tutoré, for helpful discussions; N. Cougot, R. Gillet, A. Méreau and E. Giudice from the CNRS UMR6290; the Rennes FHU CAMIn team; and B. Turlin, J. Le Seyec, M. Trotard and A. Popa for providing scientific expertise. The authors acknowledge the SFR Biosit core facilities of Rennes University with the histopathology H2P2 (A. Fautrel) and cell imaging ImPACcell (R. Le Guevel) platforms, and the ARCHE animal housing facility for their help and support. The authors also thank the Genomic platform from the translational research department of the Curie Institute in Paris. This study received financial support from the following: Institut National du Cancer PAIR Melanoma program; Ouest Valorisation; Ligue National Contre le Cancer (LNCC) Départements du Grand-Ouest; Région Bretagne; University of Rennes 1; CNRS; Association Transfusion Sanguine et Biogénétique Gaétan Saleun; MEDIC Foundation; Les Amis de l’Institut Bordet; Fondation Lambeau-Marteaux; European Organisation for Research and Treatment of Cancer Tournesol program; and the Société Française de Dermatologie. Further support was provided by a ‘Ligue Nationale Contre le Cancer’ (LNCC) fellowship (E.D.-F.) and from the Région Bretagne and the LNCC Grand Ouest (A.G.; M.M.) and from Faculté des Sciences Pharmaceutiques de l’Université de Rennes 1 (M.M.). The authors are grateful to G. Lizée for providing the Mel624 cell line, to R. B. Darnell for providing Huh7.5 Drosha KO cells, to J. Wrana for pMS2 vectors and to V. J. Hearing for the anti-TYRP1 (PEP1) antibody.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.G., M.M. and M.-D.G.; methodology: D.G., M.M., L.B., A.R., A.M., N.M., M.-L.P.-M., T.M., S.C., B.F. and S.T.-D.; investigation: D.G., M.M., L.B., F.J., A.R., E.D.-F., A.M., N.M., M.-L.P.-M., T.M., S.C., A.G. and R.B.J.; formal analysis: D.G., M.M., F.J., A.R., N.M., B.M. and F.R.; writing—original draft: D.G., M.M. and M.-D.G.; funding acquisition: D.G., F.J., T.M., S.C., G.G. and M.-D.G.; resources: F.J., F.R., P.E., J.-C.M. and G.G.; supervision: D.G. and M.-D.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Moderate TYRP1 mRNA knockdown reduces melanoma cell proliferation.
(a,b) Proliferation rate of shCTR and shTYRP1 (moderate KD) 501Mel (a) or ME1402 cells (b). Cells were counted every two days during eight or ten days (n = 2 biologically independent experiments in triplicate). TYRP1 protein was detected by Western blot experiments; pictures are representative of three independent experiments. HSC70 serves as loading control. (c,d) TYRP1 knockdown in two melanoma short-term cultures (MM057 & MM165) and in Mel624 melanoma cell line. Cell density (c) and TYRP1 mRNA levels (d) have been evaluated 4 or 7 days after infection (shTYRP1) or transfection (siTYRP1). N.D. for not detected. Each histogram represents the mean of 2 or 3 biologically independent experiments (n = 2 for cell density experiments; n = 2 for MM057 & Mel624 and n = 3 for MM165 for TYRP1 mRNA level quantification). (e) TYRP1 knockdown in two melanoma cell lines expressing TYRP1 mRNA. Three different antibodies (PEP1, AB23 & G17) were used to confirm the absence of the TYRP1 protein in SKMel28-luc cells. Pictures are representative of three independent experiments. Source data are available in Supplementary Table 8 and unprocessed original blots are shown in Supplementary Fig. 7.
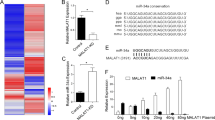
Supplementary Figure 2 miR-16 reduces TYRP1 mRNA decay induced by miR-155.
(a) Cartoon illustrating the 3′UTR of human TYRP1 with the position of the SNPs rs683 and rs910, the three MRE-155 sites (blue) and the three putative MRE-16 sites (orange). (b) MRE-155 sequences on TYRP1 3′UTR. TYRP1-C corresponds to the NM_000550.2 and TYRP1-A to NM_000550.1. Alignments have been performed using RNAhybrid34. Underlined sequences on MRE-155#2C and MRE-155#3A are detailed in boxes on the right to show the position of the SNPs in the two alleles of TYRP1. Arrows indicate the SNP rs683 and rs910 positions. (c) Effects of synthetic miR-155 on the identified regions of TYRP1 MRE-155 in 501Mel. Luciferase activity was evaluated 48 h after transfection. Each histogram represents the mean ± s.d. of n = 3 biologically independent experiments; two-sided unpaired t-test with Welch’s correction; ∗P < 0.05. (d) Northern blot quantification of miR-16 in 501Mel cells. The signal (from 501Mel cells) was fit to the standard curve from synthetic titration signals to give final copy number per cell. RNU6 served as a loading control. Pictures are representative of three experiments. (e) Northern blot quantification of TYRP1 mRNA in 501Mel cells. The signal (from 501Mel cells) was fit to the standard curve from the TYRP1 3′UTR’s synthetic titration signal to give final copy number per cell. GAPDH serves as a loading control. Picture presents three biological replicates of 501Mel. Source data are available in Supplementary Table 8.
Supplementary Figure 3 MRE-16 on human and mouse TYRP1 mRNA and biological consequences.
(a) MRE-16s’ sequences on mouse TYRP1 3′UTR (NM_031202.3). Alignments have been performed using RNAhybrid34. (b) Schematic representation of the interaction (purple base paring) of miR-16 (orange) and miR-155 (blue) with human and mouse TYRP1 MRE-16#3 and MRE-155#3 respectively. (c,d) TYRP1 knockdown in mouse B16-F10 melanoma cells using three different siRNAs. TYRP1 mRNA levels (c) and cell density (d) have been respectively evaluated at 5 and 3 days after siRNA transfection; one-way ANOVA with Holm-Sidak’s multiple comparisons test. (e) Effect on cell density of synthetic miR-16 transfected in mouse B16-F10 melanoma cells 3 days after miRNA transfection; two-sided unpaired t-test with Welch’s correction, ∗P < 0.05. Each histogram represents the mean ± s.d. (n = 3 biologically independent experiments). Source data are available in Supplementary Table 8.
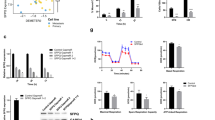
Supplementary Figure 4 TYRP1 silencing decreases expression level of several mRNAs.
(a) Workflow to identify deregulated RNAs in TYRP1 KD cells. Gene expression profile of cells transfected with three different siRNAs targeting TYRP1 or siRNA CTR. Significance analysis of microarrays was done as described in methods. siRNA efficacy for TYRP1 KD is #2 > #3 > #1. Heatmap focused on deregulated RNAs in function of siTYRP1 efficacy (top) and a z-score has been calculated using Ingenuity Pathway Analysis (IPA) (bottom). (b) mRNA expression levels of TYRP1, MAFF, NRTN, RAB17 and RasGRP3 in response to TYRP1 KD using three different siRNAs (#1-3) targeting the ORF of TYRP1 in 501Mel cells (n = 4 biologically independent experiments except for RASGRP3 expression measurement which results from n = 3 biologically independent experiments). (c) mRNA expression levels of TYRP1, MAFF, NRTN, RAB17 and RasGRP3 in response to TYRP1 KD using shTYRP1 targeting the ORF of TYRP1 in SKMel28-luc cells (n = 3 biologically independent experiments). (d) Cell density of 501Mel cells in response to siRNAs targeting MAFF, NRTN, RAB17 or RasGRP3 (n = 3 biologically independent experiments; one-way ANOVA with Holm-Sidak’s multiple comparisons test, ∗P < 0.05). Two siRNAs were used by target. (e) mRNA expression levels in response to synthetic miR-16. Each histogram represents the mean ± s.d. (n = 3 biologically independent experiments). TYRP1 and RAB17 mRNA expression in response to synthetic miR-16 are reported in Figs 2d and 4b, respectively. (f) The MRE-16s’ sequence on human MAFF and NRTN mRNAs have been identified using RNAhybrid34. For b,c and e, two-sided unpaired t-tests with Welch’s correction were done (∗P < 0.05). For b–e, values correspond to the mean ± s.d. Source data are available in Supplementary Table 8.
Supplementary Figure 5 Overall survival of patients with metastatic melanoma according to TYRP1, RAB17, NRTN mRNAs or miR-16 expression levels.
(a,b) Determination of overall survival (OS) curves by Kaplan–Meier analysis, according to the expression levels of RAB17 (a) or TYRP1 and RAB17 (b). Based on 184 skin and lymph node metastases from melanoma patients (IJB cohort). High and low expression of TYRP1 and RAB17 (a) alone were defined based on their median values and scored as 1 and 0, respectively. For combination (b), scores of TYRP1 (1 or 0) and RAB17 (1 or 0) are added and the resulting scores 1 and 2 are combined as the high score (n = 130 patients), which was significantly different from the low score 0 (n = 54 patients) regarding to OS values (two-sided Mann–Whitney test, P = 0.004). Cox regression was used to calculate P values, hazard ratios (HR) and 95% confidence intervals (CI). (c) Association of TYRP1 and RAB17 expression with patient survival. TYRP1 and RAB17 expression levels were assessed in the TCGA SKCM melanoma cohort. Patients were ranked decreasingly according to TYRP1 and RAB17 expression, resulting in almost three equal groups. The Kaplan-Meier curve representing the highest third of TYRP1 and RAB17 expressers shows a significantly lower OS as compared to the lowest third (log-rank test). (d,e) Determination of OS curves by Kaplan–Meier analysis, according to the expression levels of NRTN (d) or TYRP1 and NRTN (e). Patients were ranked decreasingly according to NRTN (d) or TYRP1 and NRTN expression (e), resulting in almost four equal groups. The Kaplan-Meier curve represents the highest and the lower quarters of expressers (log-rank test). (f,g) Determination of OS curves by Kaplan-Meier analysis, according to the expression levels of miR-16 from n = 85 patients of the IJB cohort (f) or from n = 349 patients from the TCGA cohort (g). High and low expression groups of miR-16 were defined based on its median value (log-rank test).
Supplementary Figure 6 Histological analyses of liver and kidney from PDX mice and long-term tumor growth.
(a) Representative micrographs of liver and kidney slices stained with hematoxylin from PDX-mice exposed to TSB-C1 or TSB-T3. Toxicity evaluation was blindly examined by two independent pathologists. Two mice have been showed per group among five mice analysed given similar results. Scale bar, 100 μm. (b) Tumor volume for individual PDX mice treated with TSB-C1 or TSB-T3 as described in Fig. 6b. (c) Quantification of LAMP2 mRNA in melanoma tumors treated with TSB-C1 or TSB-T3 (n = 5 mice per group). LAMP2 is not a miR-16 target. For c, lines represent the mean ± s.d.; values represent fold change relative to the mean of TSB-C1 condition; two-sided unpaired t-tests with Welch’s correction; NS, non-significant. Source data are available in Supplementary Table 8.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4459 kb)
Supplementary Table 1
Supplementary Information (XLSX 46 kb)
Supplementary Table 2
Supplementary Information (XLSX 32 kb)
Supplementary Table 3
Supplementary Information (XLSX 36 kb)
Supplementary Table 4
Supplementary Information (XLSX 42 kb)
Supplementary Table 5
Supplementary Information (XLSX 43 kb)
Supplementary Table 6
Supplementary Information (XLSX 40 kb)
Supplementary Table 7
Supplementary Information (XLSX 32 kb)
Supplementary Table 8
Supplementary Information (XLSX 46 kb)
Rights and permissions
About this article
Cite this article
Gilot, D., Migault, M., Bachelot, L. et al. A non-coding function of TYRP1 mRNA promotes melanoma growth. Nat Cell Biol 19, 1348–1357 (2017). https://doi.org/10.1038/ncb3623
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3623
This article is cited by
-
Serpine1 mRNA confers mesenchymal characteristics to the cell and promotes CD8+ T cells exclusion from colon adenocarcinomas
Cell Death Discovery (2024)
-
Oncosuppressive miRNAs loaded in lipid nanoparticles potentiate targeted therapies in BRAF-mutant melanoma by inhibiting core escape pathways of resistance
Oncogene (2023)
-
A 9‑gene expression signature to predict stage development in resectable stomach adenocarcinoma
BMC Gastroenterology (2022)
-
A novel canine reference genome resolves genomic architecture and uncovers transcript complexity
Communications Biology (2021)
-
Noncoding RNA therapeutics — challenges and potential solutions
Nature Reviews Drug Discovery (2021)