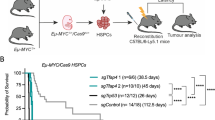
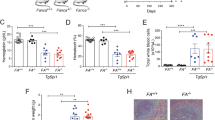
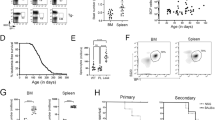
Abstract
Loss-of-function mutations of cyclic-AMP response element binding protein, binding protein (CREBBP) are prevalent in lymphoid malignancies. However, the tumour suppressor functions of CREBBP remain unclear. We demonstrate that loss of Crebbp in murine haematopoietic stem and progenitor cells (HSPCs) leads to increased development of B-cell lymphomas. This is preceded by accumulation of hyperproliferative lymphoid progenitors with a defective DNA damage response (DDR) due to a failure to acetylate p53. We identify a premalignant lymphoma stem cell population with decreased H3K27ac, which undergoes transcriptional and genetic evolution due to the altered DDR, resulting in lymphomagenesis. Importantly, when Crebbp is lost later in lymphopoiesis, cellular abnormalities are lost and tumour generation is attenuated. We also document that CREBBP mutations may occur in HSPCs from patients with CREBBP-mutated lymphoma. These data suggest that earlier loss of Crebbp is advantageous for lymphoid transformation and inform the cellular origins and subsequent evolution of lymphoid malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blobel, G. A. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood 95, 745–755 (2000).
Roelfsema, J. H. & Peters, D. J. Rubinstein-Taybi syndrome: clinical and molecular overview. Expert Rev. Mol. Med. 9, 1–16 (2007).
Kung, A. L. et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14, 272–277 (2000).
Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Papaemmanuil, E. et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122, 3616–3627 (2013).
Mullighan, C. G. et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471, 235–239 (2011).
Pasqualucci, L. et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 471, 189–195 (2011).
Okosun, J. et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 46, 176–181 (2014).
Vicente, C. et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica 100, 1301–1310 (2015).
da Silva Almeida, A. C. et al. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat. Genet. 47, 1465–1470 (2015).
Green, M. R. et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc. Natl Acad. Sci. USA 112, E1116–E1125 (2015).
Green, M. R. et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 121, 1604–1611 (2013).
Kelly, P. N., Dakic, A., Adams, J. M., Nutt, S. L. & Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 317, 337 (2007).
Kuppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 5, 251–262 (2005).
Huntly, B. J. & Gilliland, D. G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 5, 311–321 (2005).
Kikushige, Y. et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell 20, 246–259 (2011).
Chung, S. S. et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci. Transl. Med. 6, 238ra271 (2014).
Damm, F. et al. Acquired initiating mutations in early hematopoietic cells of CLL patients. Cancer Discov. 4, 1088–1101 (2014).
Weigert, O. & Weinstock, D. M. The evolving contribution of hematopoietic progenitor cells to lymphomagenesis. Blood 120, 2553–2561.
Kang-Decker, N. et al. Loss of CBP causes T cell lymphomagenesis in synergy with p27Kip1 insufficiency. Cancer Cell 5, 177–189 (2004).
Chan, W. I. et al. The transcriptional coactivator Cbp regulates self-renewal and differentiation in adult hematopoietic stem cells. Mol. Cell. Biol. 31, 5046–5060 (2011).
Xu, W. et al. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood 107, 4407–4416 (2006).
Wang, H. C., Perry, S. S. & Sun, X. H. Id1 attenuates Notch signaling and impairs T-cell commitment by elevating Deltex1 expression. Mol. Cell. Biol. 29, 4640–4652 (2009).
Jiang, Y. et al. CREBBP inactivation promotes the development of HDAC3 dependent lymphomas. Cancer Discov. 7, 38–53 (2017).
Morin, R. D. et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 42, 181–185 (2010).
Baron, B. W. et al. GFI1B, EVI5, MYB–additional genes that cooperate with the human BCL6 gene to promote the development of lymphomas. Blood Cells Mol. Dis. 52, 68–75 (2014).
Wang, X., Huang, H. & Young, K. H. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging 7, 1032–1049 (2015).
Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).
Li, H. et al. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; and ARID1A underlying the pathogenesis of follicular lymphoma. Blood 123, 1487–1498 (2014).
Prasad, M. A. et al. Ebf1 heterozygosity results in increased DNA damage in pro-B cells and their synergistic transformation by Pax5 haploinsufficiency. Blood 125, 4052–4059 (2015).
Garcia-Ramirez, I. et al. Crebbp loss cooperates with Bcl2 over-expression to promote lymphoma in mice. Blood 129, 2645–2656 (2017).
Oricchio, E. et al. Frequent disruption of the RB pathway in indolent follicular lymphoma suggests a new combination therapy. J. Exp. Med. 211, 1379–1391 (2014).
Lohr, J. G. et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl Acad. Sci. USA 109, 3879–3884 (2012).
Morin, R. D. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303 (2011).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Ogiwara, H. & Kohno, T. CBP and p300 histone acetyltransferases contribute to homologous recombination by transcriptionally activating the BRCA1 and RAD51 genes. PLoS ONE 7, e52810.
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997).
Tang, Y., Zhao, W., Chen, Y., Zhao, Y. & Gu, W. Acetylation is indispensable for p53 activation. Cell 133, 612–626 (2008).
Bashford-Rogers, R. J. et al. Network properties derived from deep sequencing of human B-cell receptor repertoires delineate B-cell populations. Genome Res. 23, 1874–1884 (2013).
Rickert, R. C., Roes, J. & Rajewsky, K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25, 1317–1318 (1997).
Zhang, J. et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B-cell lymphoma. Cancer Discov. 7, 322–337 (2017).
Goodman, R. H. & Smolik, S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 (2000).
Weigert, O. et al. Molecular ontogeny of donor-derived follicular lymphomas occurring after hematopoietic cell transplantation. Cancer Discov. 2, 47–55 (2012).
Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Quivoron, C. et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20, 25–38 (2011).
Couronne, L., Bastard, C. & Bernard, O. A. TET2 and DNMT3A mutations in human T-cell lymphoma. N. Engl. J. Med. 366, 95–96 (2012).
Green, M. R. et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat. Commun. 5, 3904 (2014).
Zhang, J. et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med. 21, 1190–1198 (2015).
Ortega-Molina, A. et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 21, 1199–1208 (2015).
Giotopoulos, G. et al. A novel mouse model identifies cooperating mutations and therapeutic targets critical for chronic myeloid leukemia progression. J. Exp. Med. 212, 1551–1569 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Stark, R. & Brown, G. DiffBind: differential binding analysis of ChIP-Seq peak data. Bioconductor http://bioconductor.org/packages/release/bioc/html/DiffBind.html (2011).
Thomas, R. K. et al. High-throughput oncogene mutation profiling in human cancer. Nat. Genet. 39, 347–351 (2007).
Fischer, A., Illingworth, C. J., Campbell, P. J. & Mustonen, V. EMu: probabilistic inference of mutational processes and their localization in the cancer genome. Genome Biol. 14, R39 (2013).
Watson, S. J. et al. Viral population analysis and minority-variant detection using short read next-generation sequencing. Phil. Trans. R. Soc. B 368, http://dx.doi.org/10.1098/Rstb.2012.0205 (2013).
Lefranc, M. P. et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 37, D1006–D1012 (2009).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Lefranc, M. P. IMGT unique numbering for the variable (V), constant (C), and groove (G) domains of IG, TR, MH, IgSF, and MhSF. Cold Spring Harb. Protoc. 2011, 633–642 (2011).
Mirzoeva, O. K. & Petrini, J. H. DNA damage-dependent nuclear dynamics of the Mre11 complex. Mol. Cell. Biol. 21, 281–288 (2001).
Acknowledgements
The Huntly laboratory is funded by the ERC (grant 647685 COMAL), KKLF, MRC, Bloodwise, the Wellcome Trust (WT) and the Cambridge NIHR BRC. We acknowledge the WT/MRC centre grant (097922/Z/11/Z) and support from WT strategic award 100140. D.S. is a Postdoctoral Fellow of the Mildred-Scheel Organisation, German Cancer Aid. We thank C. Cossetti, M. Maj and R. Schulte at the CIMR Flow Cytometry Core for their help with cell sorting. This research was supported by the Cambridge NIHR BRC Cell Phenotyping Hub. E.L. is funded by a Sir Henry Dale fellowship from the WT/Royal Society.
Author information
Authors and Affiliations
Contributions
S.J.H. designed and performed experiments, analysed data and wrote the paper. G.G. performed FACS, serial replating and transplantation experiments. H.Y. performed ChIP-Seq and qRT-PCR. S.V. performed bioinformatics analyses. O.S. maintained the mouse lines and performed experiments. R.B.-R. performed BCR amplification, sequencing and analysis. M.R. performed exome sequencing and bioinformatics analyses. A.C. designed and performed the allele-specific PCR. D.S. performed western blotting. L.Y. performed flow cytometry. W.-I.C., H.O., F.B., P.G., A.E., S.F. and T.S. provided technical assistance. N.B. and P.M. provided the CD19-Cre mice. A.S. optimized immunofluorescence experiments. W.Z. performed immunohistochemistry. A.P.H. and E.L. helped with design and sorting of HSPCs from patients. J.O. and J.F. provided patient samples. D.H. and K.G.S. designed experiments; P.W. analysed histology and immunohistochemistry. A.C. and M.Q.D. designed and analysed allele-specific PCR. D.J.A. analysed exome sequencing data. B.J.P.H. designed and analysed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Additional information for lymphoid phenotype and characterization of the IL7Rα + population in Mx-Crebbp−/− mice.
(a) Representative flow plots gated on Lin− IL7Ra+ cells demonstrating a decreased CLP compartment in Mx1-Crebbp−/− compared to WT mice. (b) However, when assessed compared to all live cells, no significant alterations in the CLP population or downstream B cell compartments were detected between genotypes (by 2-sided t-test). (c) At the early time point of assessment (8–10 weeks post pIpC), both B- and T-cells are non-significantly decreased in Mx-Crebbp−/− mice (by 2-sided t-test). (d) No significant differences in the frequency (left panel) or cell number (right panel) of early and late B cell subsets in the blood of Mx-Crebbp−/− and WT mice (by 2-sided t-test). All box plots in panels b–d represent the median with interquartile range and whiskers show the minimum and maximum values, n = 6 mice per genotype; 3 mice per genotype were quantified in 2 independent experiments. (e) The IL7-Rα + population demonstrates both lymphoid (left panel) and to a lesser extent myeloid potential (right panel), however genotype specific differences between Mx-Crebbp−/− and WT mice in terms of proliferation and self-renewal are only seen under lymphoid conditions. Box plots show the median with interquartile range and whiskers show the minimum and maximum values. For lymphoid assays, n = 6 mice per genotype; 2 mice per genotype quantified in 3 independent experiments (round 1 ∗∗∗p = 0.0002 and round 3 ∗p = 0.0181, all by 2-sided t-test). For myeloid assays, n = 4 mice per genotype; 2 mice per genotype quantified in 2 independent experiments. Photomicrograph inserts in left panel show that Crebbp−/− IL7Ra+ cells produce larger colonies per plating (representative 3rd round colonies per genotype, scale bar 50 μM) in lymphoid conditions. (f) Further GSEA of the differentially expressed dataset between Mx-Crebbp−/− and WT IL7-Rα+ progenitors demonstrates enrichment for ES core, HSC and proliferative phenotypes.
Supplementary Figure 2 Gating strategy for pre-malignant cells and next generation sequencing strategy for clonal IgH repertoire analysis.
(a) Example of the gating strategy used for flow cytometry experiments. The specific strategy used to isolate B220 + Mac1 + cells from the spleen of pre-malignant mice in Fig. 3c is shown. (b) Schema of the PCR strategy used to sequence the IgH repertoire in individual mice. Multiple upstream IgH and specific J specific primers (as exemplified by G1 and G2 in the illustrated gel in b) were used to amplify the clonal IgH rearrangements, prior to adapter ligation, NGS library preparation and 300-bp paired-end sequencing on a Mi-Seq analyser. (c) Representative gels showing amplification of peripheral blood and tumour tissue from a number of animals.
Supplementary Figure 3 Transcriptional and epigenetic abnormalities in the evolution of Crebbp−/− Lymphoma.
(a) Western blot demonstrates that, although the levels are variable, both the Crebbp−/− LPD/Lymphomas demonstrate a global decrease in H3K27Ac by comparison with the WT lymphomas. Unprocessed scans of blots are shown in Supplementary Fig. 8. (b) IGV screenshot for the Ebf1 locus, which demonstrates the downregulation of gene expression in the Crebbp−/− LPD in comparison to WT-B-cells, as well as the loss of H3K27Ac at two intragenic enhancer regions (boxed) that demonstrate Crebbp binding in normal B-cells.
Supplementary Figure 4 Genomic landscape and mutational pattern of Crebbp−/− LPD/Lymphoma.
(a) Predicted mutations in the top most recurrently altered genes and three genes known to be mutated in human lymphomas (Gna13, Card11 and Hist1h1d) are shown across our cohort of 27 samples. The samples are lymphomas/LPD unless otherwise stated (PM = premalignant B220+/Mac1Int cells, LSK = lin− Sca1+ cKit+ HSPC) and are all Crebbp−/− unless designated WT. (b) Western blotting demonstrates phosphorylation of Erk1/2 in both WT and Crebbp−/− lymphomas and phosphorylation of Stat3 in Crebbp−/− LPD 2. No activation of these pathways are demonstrated in normal WT B-cells (WT B220 + cells). Unprocessed scans of blots are shown in Supplementary Fig. 8. Comparisons of the pattern of mutations identified in Crebbp−/− deficient tumours by exome sequencing with human signatures of mutation by (c)—hierarchical clustering and (d)—Cosine correlation coefficient analysis. Both analyses demonstrate the highest correlation with Signature 1 (highlighted in red boxes). Signature 1 is associated with ageing.
Supplementary Figure 5 Epigenetic priming upon Crebbp loss.
(a) Table showing the number of genes up and downregulated along the continuum of lymphoma evolution in Mx-Crebbp−/− mice, along with downregulated differential H3K27Ac peaks at the two timepoints tested (the IL7-Rα + progenitors and the Crebbp−/− LPD), and the overlap with downregulated genes as a correlation of the epigenetic and transcriptional abnormalities. These demonstrate the sequential increase in differential gene expression and suggest that epigenetic changes precede gene expression changes. (b) Longitudinal comparisons of RNA-Seq and H3K27Ac peaks are shown for IL7-Rα + progenitors from Mx-Crebbp−/− and WT mice and for Crebbp−/− lymphomas and WT B-cells at the ‘classical’ MHC Class II locus. At the early time point in the IL7-Rα + progenitors (upper 4 tracings), no differences in expression level by RNA-Seq are seen for any of the genes shown (H2-0b, H2-Ab1, H2-Aa, H2-Eb1, H2-Eb2 or the pseudogene H2-Ea-ps), although significant loss of H3K27Ac is already observed at some loci (boxed in black). At the later timepoint, previous acetylation decreases remain and appear comparatively accentuated (in black again), however novel changes are also seen (boxed in red), including a decreased binding across the whole superenhancer between H2-Aa and H2-Eb1. These epigenetic alterations translate into more wide ranging downregulation of multiple genes in the LPD/lymphomas including H2-Ob, H2-Ab1, H2-Eb2 and H2-Ea-ps. These and other data (Fig. 2 and Supplementary Fig. 3) suggest that early loss of Crebbp generates acetylation changes that immediately alter gene expression at only a few loci. However, early loss of acetylation appears to ‘prime’ certain other loci for gene downregulation at later timepoints. We speculate that the effects of other mutations, acquired at least in part through the DDR defect associated with Crebbp loss, may contribute to this later dysregulation.
Supplementary Figure 6 Further comparisons of CD19-Crebbp−/− and WT mice.
(a) PCR analysis of efficiency of excision of Crebbp in Cd19-Crebbp−/− and Mx-Crebbp−/− mice. (b) Percentage of B-cells in the peripheral blood of Cd19-Crebbp−/− mice versus WT (c) CFC generated from IL7-Rα + progenitors under lymphoid conditions in comparison to WT mice. (d) Proportion of CLP in BM (left panel) and pro-B cells in the spleen between Cd19-Crebbp−/− and WT mice (∗p = 0.036 by 2-sided t-test, Supplementary Table 26). (e) Frequency (left panel) and cell number (right panel) of early and late B cell subsets in the blood of Cd19-Crebbp−/− and WT mice. All box-plots in panels b–e show the median and interquartile range and whiskers represent maximum and minimum values, n = 6 mice per genotype; 3 mice per genotype quantified in 2 independent experiments. (f) Aberrant B220+/MacInt populations are detected in Cd19-Crebbp−/− (47 mice) at an increased frequency over WT (41 mice).
Supplementary Figure 7 Allele specific (AS) PCR strategy and confirmation.
(a) Schematic displays the two PCR assays employed to detect the CCT genomic deletion in patient number 2 that leads to the S1608delS mutation. The forward and reverse AS primers are shown with the size of the predicted forward and reverse amplicons. (b) Analytical sensitivity of AS-PCR. Serial dilutions of the tumour DNA sample (Mut) containing the c.5039_5042delCCT mutation are shown using the upstream AS-PCR assay (left panel) and downstream AS-PCR assay (right panel). −ve—Negative control, WT—wild-type. (c) Downstream AS-PCR confirming reproducible amplification of a 209 bp product in the CD34 + CD38 + progenitor population grown under myeloid conditions. (d) Confirmation of the presence of the CCT deletion (boxed) in the AS-PCR products generated from both the upstream and downstream assays by Sanger sequencing.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1051 kb)
Supplementary Table 1
Supplementary Information (XLSX 12 kb)
Supplementary Table 2
Supplementary Information (XLSX 10 kb)
Supplementary Table 3
Supplementary Information (XLSX 10 kb)
Supplementary Table 4
Supplementary Information (XLSX 20 kb)
Supplementary Table 5
Supplementary Information (XLSX 395 kb)
Supplementary Table 6
Supplementary Information (XLSX 78 kb)
Supplementary Table 7
Supplementary Information (XLSX 658 kb)
Supplementary Table 8
Supplementary Information (XLSX 289 kb)
Supplementary Table 9
Supplementary Information (XLSX 17 kb)
Supplementary Table 10
Supplementary Information (XLSX 28 kb)
Supplementary Table 11
Supplementary Information (XLSX 20 kb)
Supplementary Table 12
Supplementary Information (XLSX 13 kb)
Supplementary Table 13
Supplementary Information (XLSX 9 kb)
Supplementary Table 14
Supplementary Information (XLSX 9 kb)
Supplementary Table 15
Supplementary Information (XLSX 126 kb)
Supplementary Table 16
Supplementary Information (XLSX 92 kb)
Supplementary Table 17
Supplementary Information (XLSX 9 kb)
Supplementary Table 18
Supplementary Information (XLSX 11 kb)
Supplementary Table 19
Supplementary Information (XLSX 29 kb)
Supplementary Table 20
Supplementary Information (XLSX 9 kb)
Supplementary Table 21
Supplementary Information (XLSX 55 kb)
Supplementary Table 22
Supplementary Information (XLSX 326 kb)
Supplementary Table 23
Supplementary Information (XLSX 10 kb)
Supplementary Table 24
Supplementary Information (XLSX 9 kb)
Supplementary Table 25
Supplementary Information (XLSX 8 kb)
Supplementary Table 26
Supplementary Information (XLSX 63 kb)
Supplementary Table 27
Supplementary Information (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Horton, S., Giotopoulos, G., Yun, H. et al. Early loss of Crebbp confers malignant stem cell properties on lymphoid progenitors. Nat Cell Biol 19, 1093–1104 (2017). https://doi.org/10.1038/ncb3597
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3597
This article is cited by
-
Identification of the effects of COVID-19 on patients with pulmonary fibrosis and lung cancer: a bioinformatics analysis and literature review
Scientific Reports (2022)
-
Pathophysiology of chronic lymphocytic leukemia and human B1 cell development
International Journal of Hematology (2020)
-
Follicular lymphoma
Nature Reviews Disease Primers (2019)
-
Genetic alterations and their clinical implications in DLBCL
Nature Reviews Clinical Oncology (2019)
-
Genetic modification of primary human B cells to model high-grade lymphoma
Nature Communications (2019)