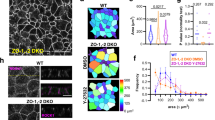
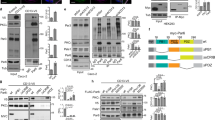
Abstract
Polarized epithelia develop distinct cell surface domains, with the apical membrane acquiring characteristic morphological features such as microvilli. Cell polarization is driven by polarity determinants including the evolutionarily conserved partitioning-defective (PAR) proteins that are separated into distinct cortical domains. PAR protein segregation is thought to be a consequence of asymmetric actomyosin contractions. The mechanism of activation of apically polarized actomyosin contractility is unknown. Here we show that the Cdc42 effector MRCK activates myosin-II at the apical pole to segregate aPKC–Par6 from junctional Par3, defining the apical domain. Apically polarized MRCK-activated actomyosin contractility is reinforced by cooperation with aPKC–Par6 downregulating antagonistic RhoA-driven junctional actomyosin contractility, and drives polarization of cytosolic brush border determinants and apical morphogenesis. MRCK-activated polarized actomyosin contractility is required for apical differentiation and morphogenesis in vertebrate epithelia and Drosophila photoreceptors. Our results identify an apical origin of actomyosin-driven morphogenesis that couples cytoskeletal reorganization to PAR polarity signalling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mellman, I. & Nelson, W. J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9, 833–845 (2008).
Sauvanet, C., Wayt, J., Pelaseyed, T. & Bretscher, A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu. Rev. Cell Dev. Biol. 31, 593–621 (2015).
Zihni, C., Mills, C., Matter, K. & Balda, M. S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 17, 564–580 (2016).
Cowan, C. R. & Hyman, A. A. Acto-myosin reorganization and PAR polarity in C. elegans. Development 134, 1035–1043 (2007).
St Johnston, D. & Ahringer, J. Cell polarity in eggs and epithelia: parallels and diversity. Cell 141, 757–774 (2010).
Morais-de-Sa, E., Mirouse, V. & St Johnston, D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 (2010).
Walther, R. F. & Pichaud, F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr. Biol. 20, 1065–1074 (2010).
Hird, S. N. & White, J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 121, 1343–1355 (1993).
Cheeks, R. J. et al. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 14, 851–862 (2004).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Mayer, M., Depken, M., Bois, J. S., Julicher, F. & Grill, S. W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010).
Bois, J. S., Julicher, F. & Grill, S. W. Pattern formation in active fluids. Phys. Rev. Lett. 106, 028103 (2011).
Goehring, N. W. et al. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science 334, 1137–1141 (2011).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633–644 (2007).
Terry, S. J. et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 13, 159–166 (2011).
Nakajima, H. & Tanoue, T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J. Cell Biol. 195, 245–261 (2011).
Zihni, C. et al. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J. Cell Biol. 204, 111–127 (2014).
Mooseker, M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu. Rev. Cell Biol. 1, 209–241 (1985).
Castillo, A. M., Lagunes, R., Urban, M., Frixione, E. & Meza, I. Myosin II-actin interaction in MDCK cells: role in cell shape changes in response to Ca2+ variations. J. Muscle Res. Cell Motil. 19, 557–574 (1998).
St Johnston, D. & Sanson, B. Epithelial polarity and morphogenesis. Curr. Opin. Cell Biol. 23, 540–546 (2011).
Unbekandt, M. & Olson, M. F. The actin-myosin regulatory MRCK kinases: regulation, biological functions and associations with human cancer. J. Mol. Med. (Berl) 92, 217–225 (2014).
Reifegerste, R. & Moses, K. Genetics of epithelial polarity and pattern in the Drosophila retina. Bioessays 21, 275–285 (1999).
Pocha, S. M. & Knust, E. Complexities of Crumbs function and regulation in tissue morphogenesis. Curr. Biol. 23, R289–R293 (2013).
Walther, R. F., Nunes de Almeida, F., Vlassaks, E., Burden, J. J. & Pichaud, F. Pak4 is required during epithelial polarity remodeling through regulating AJ stability and bazooka retention at the ZA. Cell Rep. 15, 45–53 (2016).
Gontang, A. C., Hwa, J. J., Mast, J. D., Schwabe, T. & Clandinin, T. R. The cytoskeletal regulator Genghis khan is required for columnar target specificity in the Drosophila visual system. Development 138, 4899–4909 (2011).
Hong, L. et al. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J. Biol. Chem. 288, 8531–8543 (2013).
Laprise, P. et al. Yurt, Coracle, Neurexin IV and the Na+, K+-ATPase form a novel group of epithelial polarity proteins. Nature 459, 1141–1145 (2009).
Benink, H. A. & Bement, W. M. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–439 (2005).
Robertson, F., Pinal, N., Fichelson, P. & Pichaud, F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development 139, 3432–3441 (2012).
Unbekandt, M. et al. A novel small-molecule MRCK inhibitor blocks cancer cell invasion. Cell Commun. Signal. 12, 54 (2014).
Bond, L. M., Tumbarello, D. A., Kendrick-Jones, J. & Buss, F. Small-molecule inhibitors of myosin proteins. Future Med. Chem. 5, 41–52 (2013).
Hoege, C. & Hyman, A. A. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat. Rev. Mol. Cell Biol. 14, 315–322 (2013).
Zihni, C., Balda, M. S. & Matter, K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 127, 3401–3413 (2014).
Viswanatha, R., Bretscher, A. & Garbett, D. Dynamics of ezrin and EBP50 in regulating microvilli on the apical aspect of epithelial cells. Biochem. Soc. Trans. 42, 189–194 (2014).
Viswanatha, R., Ohouo, P. Y., Smolka, M. B. & Bretscher, A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199, 969–984 (2012).
Escudero, L. M., Bischoff, M. & Freeman, M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev. Cell 13, 717–729 (2007).
Corrigall, D., Walther, R. F., Rodriguez, L., Fichelson, P. & Pichaud, F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev. Cell 13, 730–742 (2007).
Matter, K., Brauchbar, M., Bucher, K. & Hauri, H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell 60, 429–437 (1990).
Matter, K., Hunziker, W. & Mellman, I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71, 741–753 (1992).
Carlton, J. G. & Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316, 1908–1912 (2007).
Benais-Pont, G. et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160, 729–740 (2003).
Balda, M. S., Garrett, M. D. & Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 160, 423–432 (2003).
Sourisseau, T. et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol. Cell Biol. 26, 2387–2398 (2006).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Stowers, R. S. & Schwarz, T. L. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152, 1631–1639 (1999).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Winter, C. G. et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 (2001).
Walther, R. F. & Pichaud, F. Immunofluorescent staining and imaging of the pupal and adult Drosophila visual system. Nat. Protoc. 1, 2635–2642 (2006).
Karagiosis, S. A. & Ready, D. F. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development 131, 725–732 (2004).
Pinal, N. et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16, 140–149 (2006).
Crawley, S. W., Mooseker, M. S. & Tyska, M. J. Shaping the intestinal brush border. J. Cell Biol. 207, 441–451 (2014).
Acknowledgements
This work was supported by the BBSRC (BB/L007584/1 and BB/N014855/1) and the Wellcome Trust (099173/Z/12/Z). Work in the F.P. laboratory, including support to E.V., was funded by an MRC grant (MC_UU_12018/3). The N2 A71 monoclonal antibodies, developed by E. Wieschaus, were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, Iowa 52242. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Author information
Authors and Affiliations
Contributions
C.Z. performed most of the vertebrate and E.V. the Drosophila experiments. All other authors performed particular subsets of experiments. C.Z., M.S.B. and K.M. designed the project and drafted the manuscript. All authors read and contributed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Quantification of microvilli induction and role of MRCK in apical morphogenesis of Caco-2 cells.
(a) Broad view of apical surface of MDCK cells by scanning electron microscopy after MRCK knockdown and after complementation with MRCKβ-flag. (b) Measurement of apical membrane brush border cluster induction using threshold function of Nikon imaging software. (c,d) Levels of microvilli induction in human Caco-2 intestinal epithelial cells following MRCK knockdown without or with complementation with MRCKβ-flag. Panel d is based on n = 3 independent experiments and shows the data points, means ± 1 s.d. (in black), the total number of cells analysed for each type of sample across all experiments, and P-values derived from t-tests.
Supplementary Figure 2 Inhibition of ROCK does not affect Dbl3-induced apical actomyosin activation.
Confocal analysis of apical pMLC activity and F-actin in MDCK cells upon induction of Dbl3 expression by tetracycline in a conditional cell line in the absence or presence of the ROCK inhibitor GSK269962. Arrowheads point to apical cortex. Panel b is based on n = 3 independent experiments and shows the data points, means ± 1 s.d. (in black), the total number of cells analysed for each type of sample across all experiments, and P-values derived from t-tests. Scale bars: 10 μm.
Supplementary Figure 3 MRCKβ expression does not rescue loss of apical Myosin-II activation and differentiation in Dbl3-depleted MDCK cells.
(a–c) Levels of Myosin phosphorylation at the apical membrane domain (A) and basal membrane (B) and F-actin during polarization and differentiation of MDCK cells following Dbl3 siRNA transfection and with constitutive expression of MRCKβ-flag. Quantifications are based on n = 3 independent experiments and show the data points, means ± 1 s.d. (in black), the total number of cells analysed for each type of sample across all experiments, and P-values derived from t-tests show means ± 1 s.d., n + 3. (d) SEM images of the effect of MRCK knock down on microvilli induction and conditional expression of Dbl3-myc. Note, MRCKβ-flag does not rescue the Dbl3 depletion-induced phenotype. (e) Time course of tetracycline-induced Dbl3-myc expression in MDCK cells. Asterisks highlight time points with clear induction of Dbl3-myc expression that were used for the functional assays. Unprocessed original scans of blots are shown in Supplementary Fig. 8. (f) Confocal z-sections showing representative images of either endogenous or constitutively expressed MRCKβ in MDCK cells following conditional tetracycline-inducible expression of Dbl3-myc. Constitutive expression of MRCKβ-flag accelerates activation of Myosin-II at the apical membrane, highlighted by white arrows. Asterisks highlight basolateral MRCK localization at earlier stages of polarization. (g) Confocal Z-sections showing representative images of pEzrinT567 localization in MDCK cells following conditional tetracycline-inducible expression of Dbl3-myc without or with constitutive expression of MRCKβ-flag. Constitutive expression of MRCKβ-flag accelerates enrichment of active Ezrin at the apical membrane domain, indicated by white arrowheads. Scale bars, 10 μ m.
Supplementary Figure 4 Cdc42 stimulates aPKC recruitment and antagonizes Rho-signalling.
(a) Conditional tetracycline-induced expression of Dbl3 in MDCK cells stimulates increased recruitment of aPKCζ. Shown are confocal xy. (b) Levels of protein expression during tetracycline-induced Dbl3-myc expression. Unprocessed original scans of blots are shown in Supplementary Fig. 8. (c) Quantification of levels of pEzrinT567D following tetracycline-induced Dbl3-myc expression. (d) Conditional tetracycline-induced expression of Dbl3 in MDCK cells stimulates loss of junctional p114RhoGEF and LULU-2. Shown are confocal z-sections. Junctions are indicated with white arrowheads. (e,f) Spontaneously polarizing MDCK cells were treated with control or Dbl3 siRNAs, or a myristoylated aPKCζ inhibitor. LULU-2 localization at tight junctions was then analysed by confocal microscopy using ZO-1 as a marker for tight junctions (indicated by arrowheads). Quantifications are based on n = 3 independent experiments and show the data points, means ± 1 s.d. (in black), the total number of cells analysed for each type of sample across all experiments, and P-values derived from t-tests. Scale bars, 10 μm.
Supplementary Figure 5 MRCK-activated Myosin-II motor activity drives apical polarization of Par6-aPKC complex.
(a) MDCK cells conditionally expressing tetracycline-inducible Dbl3-myc were analysed by confocal microscopy using co-localization software based on the Pearson’s correlation coefficient. Co-localization is calculated in grid 3 of the scatter charts. Bright green represents co-localization of aPKCζ and Par6β, which increases and is confined to the apical cortical membrane following polarization stimulated by conditional tetracycline inducible Dbl3-myc expression in MDCK cells and is dependent on Myosin-II motor activity. Coloured outlines of apical membrane domains represent calculation areas of co-localization coefficients. Scale bars: 10 μm. (b) Co-immunoprecipitation of Par6 and aPKC indicating formation of stable complexes that are independent of Myosin-II motor activity and Dbl3 expression. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
Supplementary Figure 6 Dbl3-activated Myosin-II motor activity drives apical polarization and co-localization of brush border regulators.
(a–d) MDCK cells conditionally expressing tetracycline-inducible Dbl3-myc were analysed by confocal microscopy using co-localization software based on the Pearson’s correlation coefficient. Co-localization is calculated in grid 3 of the scatter charts. Bright green represents co-localization of Ezrin and SLK or NHERF-1 and SLK, which increases and is confined to the apical membrane domain following polarization stimulated by conditional tetracycline inducible Dbl3-myc expression in MDCK cells and is dependent on Myosin-II motor activity. Coloured outlines of apical membrane domains represent calculation areas of co-localization coefficients. Scale bars: 10 μm. (e) Expression levels of differentiation markers upon Dbl3 and Myosin manipulation. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
Supplementary Figure 7 Myosin-II activation mediates gek function in pupal photoreceptors.
(a–c) Confocal sections of a pupal retinas showing wild type cells (blue nuclei) and gek mutant cells (labelled with asterisks) stained for the apical markers aPKC and Moesin, or the junctional marker Arm. (d) Quantification of the effect of mutant gek on junctional levels of Arm (measurements from wild type and neighbouring mutant cells, paired within sections; based on n = 7 animals; P value was calculated with a t-test).
Supplementary information
Supplementary Information
Supplementary Information (PDF 20219 kb)
Supplementary Table 1
Supplementary Information (XLSX 35 kb)
Induction of polarized myosin activation by Cdc42.
Apical Cdc42 activation by conditional expression of Dbl3-myc in MDCK cells constitutively expressing EGFP-MLC was induced by adding tetracycline followed by recording image stacks every 5 min. Shown are projections of 4 images derived from the apical domain. The experiment was performed five times. (AVI 3413 kb)
Rights and permissions
About this article
Cite this article
Zihni, C., Vlassaks, E., Terry, S. et al. An apical MRCK-driven morphogenetic pathway controls epithelial polarity. Nat Cell Biol 19, 1049–1060 (2017). https://doi.org/10.1038/ncb3592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3592
This article is cited by
-
RHO GTPase family in hepatocellular carcinoma
Experimental Hematology & Oncology (2022)
-
Apical–basal polarity and the control of epithelial form and function
Nature Reviews Molecular Cell Biology (2022)
-
EFA6B regulates a stop signal for collective invasion in breast cancer
Nature Communications (2021)
-
Gene transfer of MRCKα rescues lipopolysaccharide-induced acute lung injury by restoring alveolar capillary barrier function
Scientific Reports (2021)
-
The role of polarisation of circulating tumour cells in cancer metastasis
Cellular and Molecular Life Sciences (2019)