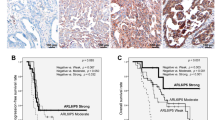
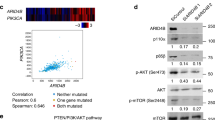
Abstract
ARID1A, encoding a subunit of the SWI/SNF chromatin-remodelling complex, is the most frequently mutated epigenetic regulator across all human cancers. ARID1A and TP53 mutations are typically mutually exclusive. Therapeutic approaches that correlate with this genetic characteristic remain to be explored. Here, we show that HDAC6 activity is essential in ARID1A-mutated ovarian cancers. Inhibition of HDAC6 activity using a clinically applicable small-molecule inhibitor significantly improved the survival of mice bearing ARID1A-mutated tumours. This correlated with the suppression of growth and dissemination of ARID1A-mutated, but not wild-type, tumours. The dependence on HDAC6 activity in ARID1A-mutated cells correlated with a direct transcriptional repression of HDAC6 by ARID1A. HDAC6 inhibition selectively promoted apoptosis of ARID1A-mutated cells. HDAC6 directly deacetylates Lys120 of p53, a pro-apoptotic post-translational modification. Thus, ARID1A mutation inactivates the apoptosis-promoting function of p53 by upregulating HDAC6. Together, these results indicate that pharmacological inhibition of HDAC6 is a therapeutic strategy for ARID1A-mutated cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Wilson, B. G. & Roberts, C. W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013).
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Jones, S. et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330, 228–231 (2010).
Wiegand, K. C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363, 1532–1543 (2010).
Chandler, R. L. et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 6, 6118 (2015).
Guan, B. et al. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J. Natl Cancer Inst. 106, dju146 (2014).
Zhai, Y. et al. Arid1a inactivation in an Apc- and Pten-defective mouse ovarian cancer model enhances epithelial differentiation and prolongs survival. J. Pathol. 238, 21–30 (2016).
Guan, B., Wang, T. L. & Shih Ie, M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 71, 6718–6727 (2011).
Guan, B., Gao, M., Wu, C. H., Wang, T. L. & Shih Ie, M. Functional analysis of in-frame indel ARID1A mutations reveals new regulatory mechanisms of its tumor suppressor functions. Neoplasia 14, 986–993 (2012).
Ye, S. et al. Clinicopathologic significance of HNF-1β, AIRD1A, and PIK3CA expression in ovarian clear cell carcinoma: a tissue microarray study of 130 cases. Medicine (Baltimore) 95, e3003 (2016).
Kobel, M. et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 29, 203–211 (2010).
Chan, J. K. et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 109, 370–376 (2008).
Mackay, H. J. et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int. J. Gynecol. Cancer 20, 945–952 (2010).
Saito, T. & Katabuchi, H. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient annual report for 2013 and treatment annual report for 2008. J. Obstet. Gynaecol. Res. 42, 1069–1079 (2016).
Li, Y., Shin, D. & Kwon, S. H. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 280, 775–793 (2013).
Bazzaro, M. et al. Ubiquitin proteasome system stress underlies synergistic killing of ovarian cancer cells by bortezomib and a novel HDAC6 inhibitor. Clin. Cancer Res. 14, 7340–7347 (2008).
Santo, L. et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY1215, in combination with bortezomib in multiple myeloma. Blood 119, 2579–2589 (2012).
Bitler, B. G. et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 21, 231–238 (2015).
Cheung, H. W. et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc. Natl Acad. Sci. USA 108, 12372–12377 (2011).
Kawaguchi, Y. et al. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115, 727–738 (2003).
Marks, P. A. Discovery and development of SAHA as an anticancer agent. Oncogene 26, 1351–1356 (2007).
Haggarty, S. J., Koeller, K. M., Wong, J. C., Grozinger, C. M. & Schreiber, S. L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl Acad. Sci. USA 100, 4389–4394 (2003).
Strasser, A., O’Connor, L. & Dixit, V. M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Stany, M. P. et al. Identification of novel therapeutic targets in microdissected clear cell ovarian cancers. PLoS ONE 6, e21121 (2011).
Raab, J. R., Resnick, S. & Magnuson, T. Genome-wide transcriptional regulation mediated by biochemically distinct SWI/SNF complexes. PLoS Genet. 11, e1005748 (2015).
Hai, Y. & Christianson, D. W. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nat. Chem. Biol. 12, 741–747 (2016).
Sykes, S. M. et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell 24, 841–851 (2006).
Sykes, S. M., Stanek, T. J., Frank, A., Murphy, M. E. & McMahon, S. B. Acetylation of the DNA binding domain regulates transcription-independent apoptosis by p53. J. Biol. Chem. 284, 20197–20205 (2009).
Mellert, H. S. & McMahon, S. B. Biochemical pathways that regulate acetyltransferase and deacetylase activity in mammalian cells. Trends Biochem. Sci. 34, 571–578 (2009).
Tang, Y., Luo, J., Zhang, W. & Gu, W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell 24, 827–839 (2006).
Seidel, C., Schnekenburger, M., Dicato, M. & Diederich, M. Histone deacetylase 6 in health and disease. Epigenomics 7, 103–118 (2015).
Chen, X., Wong, J. Y., Wong, P. & Radany, E. H. Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis. Mol. Cancer Res. 9, 448–461 (2011).
Putcha, P. et al. HDAC6 activity is a non-oncogene addiction hub for inflammatory breast cancers. Breast Cancer Res. 17, 149 (2015).
Cho, K. R. & Shih Ie, M. Ovarian cancer. Annu. Rev. Pathol. 4, 287–313 (2009).
Debnath, J., Muthuswamy, S. K. & Brugge, J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Aird, K. M. et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 3, 1252–1265 (2013).
Bitler, B. G. et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 71, 6184–6194 (2011).
McCarty, K. S. Jr et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 46, 4244s–4248s (1986).
Gao, X. et al. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc. Natl Acad. Sci. USA 105, 6656–6661 (2008).
Scarlett, U. K. et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J. Exp. Med. 209, 495–506 (2012).
Acknowledgements
We thank C. Kadoch for the ARID1A CRISPR plasmid and K. Payne and T. Fukumoto for technical assistance. This work was supported by US National Institutes of Health grants (R01CA160331, R01CA163377 and R01CA202919 to R.Z., K99CA194318 to B.G.B., K99CA194309 to K.M.A. and R01GM49758 to D.W.C.), US Department of Defense (OC140632P1 and OC150446 to R.Z.), an Ovarian Cancer Research Fund (OCRF) program project (to R.Z.) and The Jayne Koskinas & Ted Giovanis Breast Cancer Research Consortium at Wistar (to R.Z.). Support of Core Facilities was provided by Cancer Centre Support Grant (CCSG) CA010815 to The Wistar Institute.
Author information
Authors and Affiliations
Contributions
B.G.B., S.W., P.H.P., Y.H., K.M.A., Y.W. and A.V.-A. performed the experiments, and analysed data. B.G.B. and R.Z. designed the experiments. A.V.K. performed the bioinformatics analysis. F.J.R.III, J.R.C.-G., W.Z. and D.W.S. participated in the experimental design; K.R.C. and Y.Z. contributed key reagents. D.G.H., D.W.C. and R.Z. supervised studies. B.G.B., K.M.A., Y.W., K.R.C., D.W.C. and R.Z. wrote the manuscript. R.Z. conceived the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 ARID1A-inactivated cells are more sensitive to HDAC6 inhibition.
(a) ARID1A wild-type RMG1 cells with or without ARID1A knockdown were transduced with lentivirus encoding shRNA to each of the 11 individual HDACs. RNA was isolated from the indicated cells and subjected to qRT-PCR for the indicated HDACs. n = 3 independent experiments. (b) Confirmation of HDAC6 knockdown by qRT-PCR in OVCA429 cells. n = 6 independent experiments. (c) HDAC6 knockdown is selective against ARID1A-mutated clear cell or endometrioid ovarian cancer cell lines based on the Project Achilles database. The whiskers of the boxplot represent minima to maxima of the relative growth of cell lines with (n = 5 cell lines) or without ARID1A mutation (n = 11 cell lines). The box represents median bar with the first and the third quartiles. (d) Expression of HDAC6 determined by immunoblot in a panel of ovarian clear cell carcinoma cell lines with known ARID1A mutational status without or with HDAC6 knockdown using two individual shHDAC6s. GAPDH expression was used as a loading control. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6. Unprocessed original scans of all blots with size marker are shown in Supplementary Fig. 9.
Supplementary Figure 2 BRG knockdown does not affect sensitivity to HDAC6 inhibition.
(a,b) Expression of HDAC6, FLAG and loading control β-actin in ARID1A-mutated OVISE cells with knocking down of endogenous HDAC6 expression using a shRNA that targets the 3’ UTR region of the human HDAC6 gene and concurrent expression of FLAG-tagged shRNA resistant wildtype HDAC6 or a catalytically inactivated H216/611A mutant (a). The indicated cells were subjected to colony formation assay and integrated density was measured with NIH Image J software as a surrogate for cell growth (b). n = 4 independent experiments. (c) ARID1A wildtype RMG1 cells with or without ARID1A knockdown were determined for Vorinostat dose responsive curves in a 12-day colony formation assay, n = 3 independent experiments. (d–f) ARID1A wildtype RMG1 cells with or without BRG1 knockdown were examined for expression of BRG1, HDAC6 or a loading control GAPDH by immunoblot (d); examined for HDAC6 mRNA expression by qRT-PCR (e), n = 3 independent experiments; or determined for ACY1215 dose responsive curves in a 12-day colony formation assay (f), n = 4 independent experiments. (g–i) BRM compensates for the knockdown of BRG1 at the HDAC6 gene promoter. ARID1A wildtype RMG1 cells were infected with the indicated shBRG1 or shControl. Expression of BRG1, BRM1, ARID1A and a loading control β-actin was determined by immunoblot (g). The indicated cells were subjected to ChIP analysis for the HDAC6 gene promoter using antibodies against BRG1 (h) or BRM (i). An isotype matched IgG was used as a control. n = 4 independent experiments. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6. Unprocessed original scans of all blots with size marker are shown in Supplementary Fig. 9.
Supplementary Figure 3 Caspase 3, Caspase 8 and Caspase 9 knockdown in TOV21G cells.
(a) The gating strategy used for determining apoptosis based on AnnexinV-FITC and propidium iodide staining. Note that total apoptotic cells are calculated based on both early and late apoptotic fractions. (b,c) Expression of CASP3 (encodes caspase 3) mRNA (b), n = 4 independent experiments; and CASP9 (encodes caspase 9) mRNA (c), n = 3 independent experiments; determined by qRT-PCR in ARID1A-mutated TOV21G cells with or without Caspase 3 (b) or Caspase 9 knockdown (c). (d,e). Caspase 8 inhibition does not affect apoptosis induced by HDAC6 inhibition. ARID1A-mutated TOV21G cells were infected with the indicated shCaspase 8 or shControl. Relative expression of CASP8 (encodes caspase 8) was determined by qRT-PCR (d). n = 4 independent experiments. The indicated cells were treated with HDAC6 inhibitor ACY1215 (1.25 μM) or vehicle DMSO control for 72 hours and the percentages of apoptotic cells were quantified by Annexin V staining (e). n = 3 independent experiments. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6.
Supplementary Figure 4 ARID1A regulates HDAC6 expression.
(a) Expression of HDAC6, BRG1 and a loading control β-actin in ARID1A-mutated TOV21G cells with or without wildtype ARID1A restoration and with or without concurrent BRG1 knockdown. ARID1A restoration the same as Fig. 3f. (b) Relative expression of the indicated HDACs mRNA determined by qRT-PCR in ARID1A-mutated TOV21G cells with wildtype ARID1A restoration or ARID1A wildtype RMG1 cells with ARID1A knockdown. n = 3 independent experiments. ∗P < 0.01. P-value calculated via two-tailed t-test. (c) Tumour cells derived from the indicated genetic mouse endometrioid tumours were treated with ACY1215 (1.25 μM). The percentage of surviving cells was determined by colony formation. n = 3 independent experiments. (d) Relative mRNA expression of the indicated class II HDACs in ARID1A wildtype (n = 12) and mutated (n = 7) human ovarian clear cell carcinoma specimens. Note that none of them are significant. (e) ARID1A expression negatively correlates with HDAC6 expression at the mRNA level in clear cell and endometrioid ovarian cancer cells in the cell lines encyclopedia1. Pearson correlation was used for calculating P value. (f) ARID1A expression negatively correlates with HDAC6 expression at the mRNA level in a published dataset of laser capture microdissected (LCM) clear cell ovarian carcinomas and normal human ovarian surface epithelial cells2. Pearson correlation was used for calculating P value. (g) Schematic of primers’ positions for ChIP-PCR in the human HDAC6 gene locus. (h,i) ARID1A wildtype RMG1 cells with or without ARID1A knockdown were subjected to ChIP analysis using antibodies against ARID1A (h), anti-Pol II (i) or an IgG control. ChIP products were subjected to qPCR analysis using primers as indicated in (g) for the human HDAC6 gene locus. n = 4 independent experiments. (j) ARID1A wildtype RMG1 cells with or without ARID1A knockdown were subjected to ChIP analysis using an antibody against acetylated histone H3 (H3Ac) or an IgG control for the human HDAC6 gene locus. n = 4 independent experiments. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6. Unprocessed original scans of all blots with size marker are shown in Supplementary Fig. 9.
Supplementary Figure 5 p53 is required for the observed selectivity against ARID1A mutation by HDAC6 inhibitor.
(a–c) ARID1A-mutated OVISE cells with or without p53 knockdown were examined for TP53 mRNA expression by qRT-PCR (a), n = 4 independent experiments; or examined for p53 protein expression by immunoblot (b). GAPDH expression was used as a loading control; or determined for dose response curves with the indicated concentration of ACY1215 for 12 days in a colony formation assay (c). Growth inhibition was calculated based on integrated density as measured in NIH ImageJ, and values were normalized to vehicle control. n = 4 independent experiments. (d) ARID1A-mutated TOV21G cells with or without p53 knockdown were treated with the indicated concentration of CAY10603 to generate dose response curves. ARID1A wildtype RMG1 and OVCA429 were used as controls for comparison. n = 4 independent experiments. (e) ARID1A-mutated TOV21G treated with vehicle DMSO control or the HDAC6 inhibitor ACY1215 (1.25 μM). Expression of the indicated proteins was determined by immunoblot. GAPDH expression was used as a loading control. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6. Unprocessed original scans of all blots with size marker are shown in Supplementary Fig. 9.
Supplementary Figure 6 TIP60 inhibition impaired apoptosis induced by HDAC6 inhibition in ARID1A-mutated cells.
(a) ARID1A-mutated TOV21G cells were treated with vehicle control, shControl, shHDAC6, ACY1215 (1.25 μM) or CAY10603 (312 nM). RNA was extracted and utilized for next generation sequencing (RNA-seq). Expression of p53 target genes known to regulate apoptosis such as BAX, PUMA and NOXA, and known to regulate cell cycle arrest such as CDKN1A were not altered by HDAC6 inhibition in RNA-seq analysis. (b,c) ARID1A-mutated TOV21G cells were treated with the HDAC6 inhibitor ACY1215 (1.25 μM), or TIP60 inhibitor NU9056 (10 μM) or a combination. Expression of p53K120Ac and a loading control β-actin was determined by immunoblot (b). Percent apoptosis was quantified by Annexin V staining in the indicated cells (c). n = 3 independent experiments. Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. (d) The gating strategy in determining mitochondria membrane potential (MMP) by FACS analysis. Examples of maintenance of MMP and loss of MMP are shown. Statistical source data are provided in Supplementary Table 6. Unprocessed original scans of all blots with size marker are shown in Supplementary Fig. 9.
Supplementary Figure 8 HDAC6 inhibition significantly inhibits tumour growth ARID1A-mutated, but not wildtype, tumours.
(a,b) Luciferase-expressing ARID1A-mutated TOV21G cells were orthotopically transplanted into the ovarian bursa sac of SCID/nude female mice. Tumours were allowed to establish for 14 days before randomized into two groups (n = 6 mice/group). Mice were treated with vehicle control or HDAC6 inhibitor (ACY1215, 50mg/kg) daily for 21 days. Representative images of control and ACY1215 treated mice at the end of treatment (a). Total flux (photons/sec) is graphed at the indicated time points (b). ∗P = 0.0313. Error bars represent S.E.M. P-value calculated via two-tailed t-test. (c) 6-10-weeks-old Pik3caH1047R/Arid1aflox/flox female mice were intrabursally injected with adenovirus-Cre to induce clear cell ovarian carcinomas. Mice were randomized and treated with vehicle control (n = 5 mice) or ACY1215 (50 mg/kg, n = 4 mice) daily for 21 days. The changes in volumes of tumours formed on the injected ovary were calculated against the contrary side non-injected ovary from the same mice. (d–i) Luciferase-expressing ARID1A-wildtype RMG1 cells were orthotopically transplanted into the ovarian bursa sac of SCID/nude female mouse. Tumours were allowed to establish for 14 days before randomized into two groups (n = 6 mice/group). Mice were treated with vehicle control or HDAC6 inhibitor (ACY1215, 50 mg/kg) daily for 21 days. Representative images of control and ACY1215 treated mice at the end of treatment (d). At the indicated time interval during treatment, mice were imaged for luciferase expression to monitor tumour growth. Total flux (photons/sec) is graphed (e). The weight of tumours dissected from control and ACY1215 treated mice was measured at the end of treatment as a surrogate for tumour burden (f). The number of disseminated tumour nodules was counted in the indicated treatment groups (g). The serial sections of tumours dissected were subjected to immunohistochemical staining for HDAC6, Ki67, cleaved caspase 3 and p53K120Ac (h). Scale bar = 100 μm. Histological score (H-score) was calculated for 5 separate fields from 6 tumours from 6 individual mice from each of the indicated groups (i). Error bars represent mean with S.E.M. P-value calculated via two-tailed t-test. Statistical source data are provided in Supplementary Table 6.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3731 kb)
Supplementary Information
Supplementary Information (PDF 90 kb)
Supplementary Table 1
Supplementary Information (XLS 58 kb)
Supplementary Table 2
Supplementary Information (XLS 66 kb)
Supplementary Table 3
Supplementary Information (XLSX 36 kb)
Supplementary Table 4
Supplementary Information (XLS 21 kb)
Supplementary Table 5
Supplementary Information (XLSX 48 kb)
Supplementary Table 6
Supplementary Information (XLSX 84 kb)
Rights and permissions
About this article
Cite this article
Bitler, B., Wu, S., Park, P. et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol 19, 962–973 (2017). https://doi.org/10.1038/ncb3582
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3582
This article is cited by
-
PLAU promotes growth and attenuates cisplatin chemosensitivity in ARID1A-depleted non-small cell lung cancer through interaction with TM4SF1
Biology Direct (2024)
-
HDAC6 inhibitor ACY-1215 enhances STAT1 acetylation to block PD-L1 for colorectal cancer immunotherapy
Cancer Immunology, Immunotherapy (2024)
-
Treatment for ovarian clear cell carcinoma with combined inhibition of WEE1 and ATR
Journal of Ovarian Research (2023)
-
Abnormal chromatin remodeling caused by ARID1A deletion leads to malformation of the dentate gyrus
Cell Death & Differentiation (2023)
-
A comprehensive molecular analysis of 113 primary ovarian clear cell carcinomas reveals common therapeutically significant aberrations
Diagnostic Pathology (2023)