Abstract
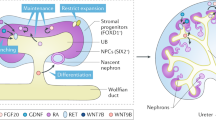
Direct reprogramming by forced expression of transcription factors can convert one cell type into another. Thus, desired cell types can be generated bypassing pluripotency. However, direct reprogramming towards renal cells remains an unmet challenge. Here, we identify renal cell fate-inducing factors on the basis of their tissue specificity and evolutionarily conserved expression, and demonstrate that combined expression of Emx2, Hnf1b, Hnf4a and Pax8 converts mouse and human fibroblasts into induced renal tubular epithelial cells (iRECs). iRECs exhibit epithelial features, a global gene expression profile resembling their native counterparts, functional properties of differentiated renal tubule cells and sensitivity to nephrotoxic substances. Furthermore, iRECs integrate into kidney organoids and form tubules in decellularized kidneys. Our approach demonstrates that reprogramming factors can be identified by targeted in silico analysis. Renal tubular epithelial cells generated ex vivo by forced expression of transcription factors may facilitate disease modelling, drug and nephrotoxicity testing, and regenerative approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Referenced accessions
ArrayExpress
Gene Expression Omnibus
References
United States Renal Data System Annual Data Report: Epidemiology of Kidney Disease in the United States (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Xia, Y. et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 15, 1507–1515 (2013).
Papadimou, E. et al. Direct reprogramming of human bone marrow stromal cells into functional renal cells using cell-free extracts. Stem Cell Rep. 4, 685–698 (2015).
Knoepfler, P. S. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27, 1050–1056 (2009).
Xu, J., Du, Y. & Deng, H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell 16, 119–134 (2015).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Najm, F. J. et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 31, 426–433 (2013).
Yang, N. et al. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 31, 434–439 (2013).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Du, Y. et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 14, 394–403 (2014).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Sekiya, S. & Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 (2011).
Buganim, Y. et al. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell 11, 373–386 (2012).
Hendry, C. E. et al. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J. Am. Soc. Nephrol. 24, 1424–1434 (2013).
Heinaniemi, M. et al. Gene-pair expression signatures reveal lineage control. Nat. Methods 10, 577–583 (2013).
Pereira, C. F., Lemischka, I. R. & Moore, K. Reprogramming cell fates: insights from combinatorial approaches. Ann. NY Acad. Sci. 1266, 7–17 (2012).
Rackham, O. J. et al. A predictive computational framework for direct reprogramming between human cell types. Nat. Genet. 48, 331–335 (2016).
Lang, A. H., Li, H., Collins, J. J. & Mehta, P. Epigenetic landscapes explain partially reprogrammed cells and identify key reprogramming genes. PLoS Comput. Biol. 10, e1003734 (2014).
Ravasi, T. et al. An atlas of combinatorial transcriptional regulation in mouse and man. Cell 140, 744–752 (2010).
Harding, S. D. et al. The GUDMAP database—an online resource for genitourinary research. Development 138, 2845–2853 (2011).
McMahon, A. P. et al. GUDMAP: the genitourinary developmental molecular anatomy project. J. Am. Soc. Nephrol. 19, 667–671 (2008).
Yu, J. et al. Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 139, 1863–1873 (2012).
Shao, X., Somlo, S. & Igarashi, P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 13, 1837–1846 (2002).
Liu, M. L. et al. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 4, 2183 (2013).
Bar-Nur, O. et al. Small molecules facilitate rapid and synchronous iPSC generation. Nat. Methods 11, 1170–1176 (2014).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795–797 (2008).
Huang, P. et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14, 370–384 (2014).
Valentich, J. D., Tchao, R. & Leighton, J. Hemicyst formation stimulated by cyclic AMP in dog kidney cell line MDCK. J. Cell. Physiol. 100, 291–304 (1979).
Cahan, P. et al. CellNet: network biology applied to stem cell engineering. Cell 158, 903–915 (2014).
Cabili, M. N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Neph, S. et al. Circuitry and dynamics of human transcription factor regulatory networks. Cell 150, 1274–1286 (2012).
Jaitovich, A. & Bertorello, A. M. Salt, Na+, K+-ATPase and hypertension. Life Sci. 86, 73–78 (2010).
Lee, J. W., Chou, C. L. & Knepper, M. A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 26, 2669–2677 (2015).
Cui, S., Verroust, P. J., Moestrup, S. K. & Christensen, E. I. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am. J. Physiol. 271, F900–F907 (1996).
Vaidya, V. S. et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 28, 478–485 (2010).
Ciarimboli, G. et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 167, 1477–1484 (2005).
Yonezawa, A. et al. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem. Pharmacol. 70, 1823–1831 (2005).
Unbekandt, M. & Davies, J. A. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 77, 407–416 (2010).
Song, J. J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 19, 646–651 (2013).
Legouis, D. et al. Ex vivo analysis of renal proximal tubular cells. BMC Cell Biol. 16, 12 (2015).
Nam, Y. J. et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development 141, 4267–4278 (2014).
Wernig, M. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 26, 916–924 (2008).
Lienkamp, S. et al. Inversin relays Frizzled-8 signals to promote proximal pronephros development. Proc. Natl Acad. Sci. USA 107, 20388–20393 (2010).
Nieuwkoop, P. & Faber, J. Normal Table of Xenopus Laevis (Daudin) (Garland Publishing, 1994).
Giles, R. H., Ajzenberg, H. & Jackson, P. K. 3D spheroid model of mIMCD3 cells for studying ciliopathies and renal epithelial disorders. Nat. Protoc. 9, 2725–2731 (2014).
Nagy, A. G., Gertsenstein, M., Vintersten, K. & Behringer, R. Manipulating the Mouse Embryo. A Laboratory Manual 3rd edn, 453–506 (Cold Spring Harbor Laboratory Press, 2003).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 44, W3–W10 (2016).
Kutner, R. H., Zhang, X. Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009).
Kaminski, M. M. et al. Direct reprogramming of mouse embryonic fibroblasts into renal tubular epithelial cells (iRECs). Nat. Protoc. Exch. http://dx.doi.org/10.1038/protex.2016.70 (2016).
Xia, Y. et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat. Protoc. 9, 2693–2704 (2014).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Acknowledgements
We thank A. Heer, A. Sammarco and B. Joch for excellent technical assistance; the staff of the SFB1140 project Z01 and R. Nitschke (Life Imaging Centre (LIC), Albert-Ludwigs-University Freiburg) for help with confocal microscopy; D. Pfeifer for performing microarrays; M. Follo for FACS analysis; O. Wessely (Cleveland, USA), P. Vize (Calgary, Canada), N. Ueno (National Institute for Basic Biology, Japan) and D. Trono (Lausanne, Switzerland) for generously providing plasmids; M. Köttgen for thoughtful comments on the manuscript. This work was supported by the Emmy Noether Programme to S.S.L. (LI1817/2-1) and S.J.A. (AR732/1-1), Projects B07 of the collaborative research initiative KIDGEM (SFB 1140) to S.S.L. and S.J.A. and project A03 of SFB 850 to S.J.A. by the German Research Foundation (DFG), BIOSS Centre of Biological Signalling Studies, and the Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School) to S.J.A.
Author information
Authors and Affiliations
Contributions
M.M.K., J.T., C.K., H.E., J.K., A.-L.M., R.P., F.G., O.K. and S.S.L. performed experiments. M.M.K., J.T., S.J.A. and S.S.L. planned and analysed experiments. G.W. and T.B.H. helped with data analysis and during final stages of the manuscript. M.M.K., J.T., S.J.A. and S.S.L. wrote and edited the manuscript. S.S.L. conceived the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the following competing financial interest exists: patent applications are pending (EPA 16152655.3 and EP16185290.0).
Integrated supplementary information
Supplementary Figure 3 Distribution of known reprogramming factors in quantitative expression data.
(a) Scatter-plots depicting the absolute and relative expression levels of transcription factors (TFs) in brain, liver, heart, testis, thymus and muscle tissue. TFs successfully used for reprogramming into the indicated cell type (blue) are highlighted in red. Dashed lines indicate the level of the 50th percentile of absolute expression and the 95th percentile of relative expression. Combinations of TFs are according to the studies referenced below. (b) Summary of Xenopus whole mount in situ hybridization experiments: Spatial expression pattern at stage 38. White, grey and black colored boxes indicate undetectable, weak and strong gene expression within the different segments of the Xenopus pronephros. PT1, PT2, PT3: proximal tubule segment 1, 2 and 3. IT1, IT2: intermediate tubule segment 1 and 2. DT1, DT2: distal tubule segment 1 and 2. CT: connecting tubule (left panel). Temporal expression pattern at stages 22, 26, 33 and 38 of Xenopus development (right panel). (c) Summary of transcription factor expression in E12-E17 mouse embryonic kidney. E12-E17 embryonic kidneys were serially sectioned and analyzed by in situ hybridization for indicated genes. Spatial expression patterns were determined within the kidney anlagen and sections analyzed for expression within the ureteric bud (ub) and metanephric mesenchyme (mm) at E12, and nephrogenic mesenchyme (nm), stroma/interstitium (str), renal vesicle/glomerulus (rv/g) and tubular epithelium (te) from E13 to E17, -: no expression.
Supplementary Figure 4 Whole mount in situ hybridization of candidate reprogramming factors in Xenopus embryos.
Whole mount in situ hybridization (WISH) of the indicated genes. WISH was performed on Xenopus embryos at stages 22, 26, 33, and 38. Enlarged views show the pronephric region. Scale bars, 500 μm.
Supplementary Figure 5 Expression of candidate reprogramming transcription factors during kidney development in mouse.
mRNA expression of the indicated 20 transcription factors was visualized by in situ hybridization on paraffin sections of mouse nephrogenic anlagen and embryonic kidneys from E12 to E17 on consecutive sections from the same embryos ensuring increased comparability between expression of different transcription factors. Scale bars, 200 μm (E12, E13), 500 μm (E14–E17).
Supplementary Figure 6 iRECs can be induced by a multicistronic lentiviral vector and can be generated from adult tail tip fibroblasts.
(a) Flow cytometry (FC) analysis of control MEFs obtained from membrane-tomato red mice (TOM CTL) and from membrane-GFP mice (GFP CTL) used for FC gate setting. MIX CTL: mixture of membrane-tomato red MEFs and membrane-GFP MEFs. (b) Vector map of the multicistronic construct showing the four reprogramming factors (Pax8, Hnf1b, Emx2, Hnf4a) separated by 2A peptide sequences. pWPXLd was used as lentiviral backbone. (c) Expression of 2A-tagged TFs was controlled using an antibody against the 2A consensus peptide motif. GFP transfected cells served as a control. (d) FC analysis of GFP+ cells in KSP-Cre reporter MEFs treated with a lentivirus harboring the multicistronic vector after 39 days of culture. (e) FC analysis for the presence of GFP+ cells in MEFs, young TTFs (P7) and adult TTFs (P56) 1 and 5 weeks after high titer lentiviral infection with 4TFs (Pax8, Emx2, Hnf4a and Hnf1b); representative FC plots are shown, percentage of GFP+ cells is indicated with standard error for the respective experiment. (f) Immunostaining of 0TF or 4TF treated adult TTFs for the indicated proteins. Scale bar, 50 μm (f).
Supplementary Figure 7 Transcriptome analysis of iRECs.
(a) Median RPKM (Reads per kilobase per million mapped reads) in human (left diagrams) and mouse (middle and right diagrams) global gene expression profiles of 10-fold upregulated and downregulated genes in iRECs compared to MEFs or primary RECs. Error bars, quantile range. (b) Median RPKM in global gene expression across renal tubular segments3 of differentially regulated genes as indicated. Error bars, quantile range. One-way ANOVA was used to determine differential gene expression in the microarray dataset. (c) Gene ontology term analysis of 10-fold upregulated genes in iRECs compared to MEFs.
Supplementary Figure 8 Heterogeneity, stability, proliferation and nephrotoxicity testing in iRECs.
(a) Flow cytometry (FC) analysis of MEFs, iRECs and IMCD-3 cells for expression of GFP and EPCAM, AQP1, and ATP1A1. The percentage of cells within each quadrant is given. (b) Co-immunostaining of iRECs for proteins with segment restricted expression as detected by FC. Proximal tubule cells were detected by LTL (Lotus tetragonolobus) staining. (c) Expression levels of the 13 candidate reprogramming factors in microdissected tubule segments of adult rats39. (d) qPCR analysis for detection of exogenous reprogramming factors. iRECs EX: generated with a LoxP containing provirus for removal of the exogenous reprogramming factors after CRE expression. iRECs: generated with a provirus lacking loxP sites for constitutive expression, n = 3 biological samples. (e) Immunostaining of iRECs and iRECs EX for the indicated proteins. (f) mRNA expression levels of the indicated genes as determined by qPCR in MEFs and iRECs EX, n = 3 biological samples. (g) Relative levels of p16 mRNA as determined by qPCR in P20 and P40 iRECs and MEFs, n = 3 biologically independent samples, ns: not significant, P > 0.05. (h) Quantification of cell death (%) in cisplatin treated MEFs (red line) and iRECs (black line) or untreated controls, n = 3 biologically independent samples. (i) FC analysis for KIM1 expression in iRECs and MEFs treated with gentamicin (1 mg ml−1), n = 3 biologically independent samples. Error bars, s.e.m., Student’s unpaired t-test, ∗∗∗P < 0.001, ∗∗P < 0.01,∗P < 0.05 (d,f–h). Scale bar, 50 μm (e).
Supplementary Figure 9 Improvement of human iREC induction efficiency and specificity.
(a) Percentage of THY-1− or EPCAM + cells in mouse and human fibroblasts after treatment with 4TF as determined by flow cytometry (FC). (b) FC analysis of the percentage of THY-1− and EPCAM+ human fibroblasts after treatment with SV40 (large T antigen), 4TF or SV40 and 4TF. (c) Percentage of CDH16-GFP+ reporter mouse fibroblasts after treatment with 4 TFs as determined by FC. Fibroblasts were transduced with a human CDH16-GFP reporter plasmid and SV40, selected for GFP cells with stable integration of the reporter and infected with 4TFs.
Supplementary Figure 10 Scans of the original Western blots as shown in Supplementary Fig. 4c.
Short (10 s) and long (1 min) exposure times are shown.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3045 kb)
Dome formation of iRECs.
Confocal 3D-stack of iREC dome formation, as depicted in Fig. 3c. iRECs express membrane GFP (green), the nuclei are stained with Hoechst (blue). (AVI 7579 kb)
Integration of iRECs into kidney organoids.
Confocal 3D-stack of reaggregated kidney organoid as depicted in Fig. 7c. iRECs express membrane GFP (green), LTL staining for renal tubular cells is depicted in magenta. (AVI 2874 kb)
iRECs form tubular structures in decellularized kidneys.
Confocal 3D-stack of tubule forming iRECs guided by the extracellular matrix of decellularized kidneys as depicted in Fig. 7f. iRECs express membrane GFP (green), the nuclei are stained with Hoechst (blue). (AVI 2334 kb)
Rights and permissions
About this article
Cite this article
Kaminski, M., Tosic, J., Kresbach, C. et al. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol 18, 1269–1280 (2016). https://doi.org/10.1038/ncb3437
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3437
This article is cited by
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy
Molecular Cancer (2023)
-
Generation of proximal tubule-enhanced kidney organoids from human pluripotent stem cells
Nature Protocols (2023)
-
Differentiated kidney tubular cell-derived extracellular vesicles enhance maturation of tubuloids
Journal of Nanobiotechnology (2022)
-
Drug toxicity in the proximal tubule: new models, methods and mechanisms
Pediatric Nephrology (2022)
-
The renal lineage factor PAX8 controls oncogenic signalling in kidney cancer
Nature (2022)