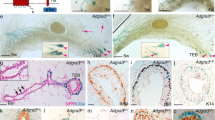
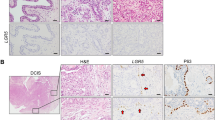
Abstract
The mammary gland is composed of a complex cellular hierarchy with unusual postnatal plasticity. The identities of stem/progenitor cell populations, as well as tumour-initiating cells that give rise to breast cancer, are incompletely understood. Here we show that Lgr6 marks rare populations of cells in both basal and luminal mammary gland compartments in mice. Lineage tracing analysis showed that Lgr6+ cells are unipotent progenitors, which expand clonally during puberty but diminish in adulthood. In pregnancy or following stimulation with ovarian hormones, adult Lgr6+ cells regained proliferative potency and their progeny formed alveoli over repeated pregnancies. Oncogenic mutations in Lgr6+ cells resulted in expansion of luminal cells, culminating in mammary gland tumours. Conversely, depletion of Lgr6+ cells in the MMTV-PyMT model of mammary tumorigenesis significantly impaired tumour growth. Thus, Lgr6 marks mammary gland progenitor cells that can initiate tumours, and cells of luminal breast tumours required for efficient tumour maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Macias, H. & Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 1, 533–557 (2012).
Inman, J. L., Robertson, C., Mott, J. D. & Bissell, M. J. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142, 1028–1042 (2015).
Rios, A. C., Fu, N. Y., Lindeman, G. J. & Visvader, J. E. In situ identification of bipotent stem cells in the mammary gland. Nature 506, 322–327 (2014).
Wang, D. et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 (2015).
van Amerongen, R., Bowman, A. N. & Nusse, R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11, 387–400 (2012).
Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011).
dos Santos, C. O. et al. Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc. Natl Acad. Sci. USA 110, 7123–7130 (2013).
Lafkas, D. et al. Notch3 marks clonogenic mammary luminal progenitor cells in vivo. J. Cell Biol. 203, 47–56 (2013).
Skibinski, A. & Kuperwasser, C. The origin of breast tumor heterogeneity. Oncogene 34, 5309–5316 (2015).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Visvader, J. E. & Stingl, J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 28, 1143–1158 (2014).
Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 (2006).
Prater, M. D. et al. Mammary stem cells have myoepithelial cell properties. Nat. Cell Biol. 16, 942–950 (2014).
Chang, T. H. et al. New insights into lineage restriction of mammary gland epithelium using parity-identified mammary epithelial cells. Breast Cancer Res. 16, R1 (2014).
Wagner, K. U. et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377–1386 (2002).
Ren, W. et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl Acad. Sci. USA 111, 16401–16406 (2014).
Oeztuerk-Winder, F., Guinot, A., Ochalek, A. & Ventura, J. J. Regulation of human lung alveolar multipotent cells by a novel p38α MAPK/miR-17-92 axis. Embo J. 31, 3431–3441 (2012).
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Füllgrabe, A. et al. Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Rep. 5, 843–855 (2015).
Lim, E. et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 12, R21 (2010).
Rodilla, V. et al. Luminal progenitors restrict their lineage potential during mammary gland development. PLoS Biol. 13, e1002069 (2015).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Kaanta, A. S., Virtanen, C., Selfors, L. M., Brugge, J. S. & Neel, B. G. Evidence for a multipotent mammary progenitor with pregnancy-specific activity. Breast Cancer Res. 15, R65 (2013).
Prat, A., Ellis, M. J. & Perou, C. M. Practical implications of gene-expression-based assays for breast oncologists. Nat. Rev. Clin. Oncol. 9, 48–57 (2012).
Goldhirsch, A. et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 22, 1736–1747 (2011).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Bamford, S. et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer 91, 355–358 (2004).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Scata, K. A. & El-Deiry, W. S. p53, BRCA1 and breast cancer chemoresistance. Adv. Exp. Med. Biol. 608, 70–86 (2007).
Wei, G., Wang, Y., Zhang, P., Lu, J. & Mao, J. H. Evaluating the prognostic significance of FBXW7 expression level in human breast cancer by a meta-analysis of transcriptional profiles. J. Cancer Sci. Ther. 4, 299–305 (2012).
Akhoondi, S. et al. Inactivation of FBXW7/hCDC4-β expression by promoter hypermethylation is associated with favorable prognosis in primary breast cancer. Breast Cancer Res. 12, R105 (2010).
Wright, K. L. et al. Ras signaling is a key determinant of metastatic dissemination and poor survival of luminal breast cancer patients. Cancer Res. 75, 4960–4972 (2015).
Wallace, M. D. et al. Comparative oncogenomics implicates the neurofibromin 1 gene (NF1) as a breast cancer driver. Genetics 192, 385–396 (2012).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992).
Yin, Y. et al. Characterization of medroxyprogesterone and DMBA-induced multilineage mammary tumors by gene expression profiling. Mol. Carcinog. 44, 42–50 (2005).
Oshima, R., Kim, S., Roopra, A. & Alexander, C. M. A phenotypic mouse model of basaloid breast tumors. PloS ONE 7, e30979 (2012).
Giraddi, R. R. et al. Stem and progenitor cell division kinetics during postnatal mouse mammary gland development. Nat. Commun. 6, 8487 (2015).
Richert, M. M., Schwertfeger, K. L., Ryder, J. W. & Anderson, S. M. An atlas of mouse mammary gland development. J. Mammary Gland Biol. Neoplasia 5, 227–241 (2000).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Visvader, J. E. & Clevers, H. Tissue-specific designs of stem cell hierarchies. Nat. Cell Biol. 18, 349–355 (2016).
Wuidart, A. et al. Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes Dev. 30, 1261–1277 (2016).
Shyamala, G., Chou, Y. C., Cardiff, R. D. & Vargis, E. Effect of c-neu/ ErbB2 expression levels on estrogen receptor α-dependent proliferation in mammary epithelial cells: implications for breast cancer biology. Cancer Res. 66, 10391–10398 (2006).
Mastroianni, M. et al. Wnt signaling can substitute for estrogen to induce division of ERα-positive cells in a mouse mammary tumor model. Cancer Lett. 289, 23–31 (2010).
Arendt, L. M. & Kuperwasser, C. Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy. J. Mammary Gland Biol. Neoplasia 20, 9–25 (2015).
Boras-Granic, K., Dann, P. & Wysolmerski, J. J. Embryonic cells contribute directly to the quiescent stem cell population in the adult mouse mammary gland. Breast Cancer Res. 16, 487 (2014).
Booth, B. W. & Smith, G. H. Estrogen receptor-α and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 8, R49 (2006).
Burga, L. N. et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 13, R30 (2011).
Tung, N. et al. Prevalence and predictors of loss of wild type BRCA1 in estrogen receptor positive and negative BRCA1-associated breast cancers. Breast Cancer Res. 12, R95 (2010).
Tung, N. et al. Estrogen receptor positive breast cancers in BRCA1 mutation carriers: clinical risk factors and pathologic features. Breast Cancer Res. 12, R12 (2010).
Molyneux, G. et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7, 403–417 (2010).
Joshi, P. A. et al. Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807 (2010).
Schramek, D. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010).
Wagner, K. U. et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10, 545–553 (2001).
Malhotra, G. K. et al. The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Dev. Biol. 14 (2014).
Koren, S. et al. PIK3CA induces multipotency and multi-lineage mammary tumours. Nature 525, 114–118 (2015).
Van Keymeulen, A. et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525, 119–123 (2015).
Bao, L., Cardiff, R. D., Steinbach, P., Messer, K. S. & Ellies, L. G. Multipotent luminal mammary cancer stem cells model tumor heterogeneity. Breast Cancer Res. 17, 137 (2015).
Chen, K., Huang, Y. H. & Chen, J. L. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol. Sin. 34, 732–740 (2013).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Liu, X. et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl Acad. Sci. USA 104, 12111–12116 (2007).
Jandke, A. et al. The F-box protein Fbw7 is required for cerebellar development. Dev. Biol. 358, 201–212 (2011).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Shehata, M., van Amerongen, R., Zeeman, A. L., Giraddi, R. R. & Stingl, J. The influence of tamoxifen on normal mouse mammary gland homeostasis. Breast Cancer Res. 16, 411 (2014).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Ewald, A. J., Brenot, A., Duong, M., Chan, B. S. & Werb, Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581 (2008).
Acknowledgements
We thank Å. Bergström, M. Saghafian and R. Kuiper for technical help and support, and E. Tüksammel for mouse husbandry, genotyping and surgery. We are grateful to H. Clevers for providing the Lgr6-EGFP-IRES-CreERT2 mouse line. We also thank P.-M. Blaas and E. Casanova for critical reading of the manuscript. Finally, we thank C. Cremona for her terrific assistance in writing the manuscript. This work was supported by grants from M. Skłodowska-Curie actions (Project no. 297639) and the Wenner Gren Foundation (to L.Blaas), the Swedish Cancer Society (to M.G., R.T.), the German Research Foundation DFG (to M.G., DFG Ge 2386/1-1), the German Academic Exchange Service DAAD (to D.B.), the Knut and Alice Wallenberg Foundation (to I.D.), the Breast Cancer Theme group at the Karolinska Institutet (to A.B.A., R.T.), the Swedish Research Council (to R.T.), the Center for Innovative Medicine in the Department of Biosciences and Nutrition, Karolinska Institutet (to R.T.), and the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001039), the UK Medical Research Council (FC001039) and the Wellcome Trust (FC001039). This study was performed partly at the Francis Crick Institute in London, partly at the Wallenberg Institute for Regenerative Medicine Flow Cytometry Facility, and Regenerative Medicine, Karolinska Institutet, Huddinge, Sweden, and partly at the Live Cell Imaging Unit/Nikon Center of Excellence in the Department of Biosciences and Nutrition, which receives funding from the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Center for Innovative Medicine and the Jonasson donation to the School of Technology and Health, Kungliga Tekniska Högskolan, Huddinge, Sweden.
Author information
Authors and Affiliations
Contributions
L.Blaas and F.P. designed and performed experiments, analysed data and wrote the manuscript. H.A.M. performed immunostaining. A.B.A. performed mouse experiments, in situ hybridization and immunostaining. E.J.R. generated the FRL mice. M.G. and A.M. performed experiments and analysed data. D.B. conducted confocal imaging and analysed data. I.D. planned and set up flow cytometry experiments. B.S.-D. and R.S. performed immunohistochemistry and in situ hybridization. R.M. performed RNA-seq analysis. L.Bhaw and A.K.S. performed RNA sequencing. J.J. provided analytical tools. G.S. performed immunopathology evaluations of mammary gland tumours. I.M. assisted with MMTV-PyMT tumour cell isolation and culture, and data interpretation. R.T. and A.B. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 FACS strategies used for detection and sorting of EGFP+ mammary epithelial cell populations in single cell suspensions from Lgr6-CreERT2+/− mammary glands at 5 weeks of age.
(a) FACS strategy applied to sort EGFP+ and EGFP− mammary epithelial cells (quadrant I) from 5-6 week old mice for RNA sequencing analysis (b) Heat map of RNA-Seq transcriptome analysis from two experiments (Ex.1/Ex.2), showing all genes differentially expressed between Lgr6+ and Lgr6− cells isolated from mammary glands of 5-6w-old Lgr6-CreERT2+/− mice. Red indicates upregulated genes; blue indicates downregulated genes. (n = 6 mice for each of the 2 biological replicates). (c) FACS strategy for determining the distribution of EGFP+ cells over basal (lin− CD29hiCD24+) and luminal (lin− CD29loCD24+) MECs. The gate for EGFP+ cells was set using wildtype mice as fluorescence-minus-one (FMO) EGFP controls.
Supplementary Figure 2 Lgr6-expressing cells in postnatal mammary gland development.
(a) K8 immunostaining of Lgr6-CreERT2+/− mammary glands at postnatal day 14 (P14) and P30. EGFP+ cells in luminal (arrowheads) and basal cells (arrows). Scale bars, 25 μm. (b) Recombination efficiency 24 h after tamoxifen administration to 2w-old (n = 5 mice) and 4w-old (n = 4 mice) Lgr6-CreERT2+/−:tdTomato+/− (LT) females. Mean ± s.e.m. (c) FACS plot showing the distribution of tdTomato+ cells over MECs 24 h after tamoxifen administration to 2w-old (left) and 4w-old (right) LT mice. (d) FACS analysis to exclude recombination of the Rosa26-tdTomato allele by Lgr6-CreERT2 in the absence of Tamoxifen. Left/middle panel: MECs from 10w old wildtype (WT) or LT mice injected with sunflower seed oil at P12 and analysed 8w later Right: Detection of tdTomato+ cells 8w after administration of 1 mg tamoxifen at P12. (e) Mammary gland from LT mouse injected with sunflower seed oil at P12 and imaged 8w later. Scale bars, 1 mm (f) F-actin staining of LT mammary glands 22w p.i. in pre-puberty (right panel) and 24w p.i. in puberty (left panel). Scale bars, 100 μm, 20 μm (insets). (g, h) Distribution of basal and luminal multi-cellular tdTomato+ clones, and cell patches spanning both compartments after induction in pre-puberty (2w, g). and in puberty (4w, h). Numbers indicate % of multicellular areas comprising adjacent basal and luminal cells. (Analysed clones pooled from n = 3 mice per timepoint: n = 1,157, n = 344, n = 542, n = 645, n = 691 for 2w, and n = 531, n = 1,476, n = 1,733, n = 163, n = 782, n = 597 for 4w). Mean ± s.e.m. (i) K14/K8 immunostained mammary gland 16w p.i. (4w) showing a rare tdTomato+ ‘clone’ spanning the basal (arrow) and luminal (arrowhead) compartments. Scale bar, 25 μm. (j) RNA in situ hybridization (ISH) analysis. Left: Lgr6 (blue arrowheads) and Axin2 (red arrowheads). Lgr6/Axin2 co-expression (arrows) can be observed in some cells. Right: Rare cells in the terminal end buds (TEBs) of pubertal mammary glands co-express Lgr6 and Lgr5 mRNA (arrows; inset). Scale bars, 10 μm (left), 20 μm (right). (k) Overview of transplantation assays of basal EGFP+ and EGFP− cells from adult virgin LT females into emptied fat pads of NOD/SCID mice. See Supplementary Table 2 for source data for g,h.
Supplementary Figure 3 Basal and luminal Lgr6 + progenitors contribute to the alveolar network during pregnancy.
(a, b) Confocal images of K14/K8 immunostained whole mount mammary glands from LT female at 17.5 dpc in first pregnancy (17.5 dpc P1). tdTomato+ clones dispersed over newly formed alveolar structures contain either basal K14+ cells (a) or K8+ alveolar cells (b). Scale bars, 40 μm (a), 20 μm (b). (c) Confocal overview image of mammary gland on day 1 of the first lactation (1d L1). LN: lymph node. Scale bar, 2 mm. (d) 3D reconstruction of 1d L1 mammary gland showing an alveolar network of myoepithelial tdTomato+ cells. Scale bar, 50 μm. (e) Quantification of alveoli containing basal and luminal tdTomato + cells in the first lactation after tamoxifen administration to LT females at 4w of age. (1,115 alveoli pooled from 3 mice were analysed). (f) Confocal z-stack image of tdTomato+ cells lining ducts and alveolar remnants (arrows), and adjacent to apoptotic cells (arrowheads) at the end of involution (21d Inv1). Autofluorescent signal (arrowheads) comes from dying cells. Scale bar, 50 μm. (g) Confocal z-stack image of K14/K8 immunostained mammary duct at the end of involution. Scale bar, 25 μm. (h) 3D reconstruction of K8 stained mammary duct showing luminal clones remaining after involution. Scale bar, 50 μm.
Supplementary Figure 4 Analysis of Lgr6-expressing cells in the adult mammary gland.
(a) Flow cytometry dot plot showing distribution of EGFP expression in MECs of 8w-old Lgr6-CreERT2+/− females. (b) Bar graph demonstrating the recombination efficiency 24 h after tamoxifen administration to 8w-old (n = 4 mice) Lgr6-CreERT2+/−:Rosa26-tdTomato+/− (LT) females. Mean ± s.e.m. (c) Flow cytometry plot showing the distribution of tdTomato-labelled cells over MECs 24 h p.i. (d) Flow cytometry dot plots of primary MECs 22w p.i., showing the distribution of tdTomato-labelled cells. (e) Representative flow cytometry plots showing very rare EGFP+ cells (left panel), present in both MEC compartments (right panel) of mammary glands from 30w-old Lgr6-CreERT2+/− females. (f) Confocal z-stack image (image 1) of K14/K8 immunostaining showing adjacent basal and luminal tdTomato+ clones (arrowheads) in 1d L1 alveoli of 8w-induced LT mice. Images 2–11 show the resolution of the 3D image into 10 single plane images. Insets show magnified regions containing adjacent basal and luminal tdTomato+ cells (arrowheads). Scale bar, 20 μm. (g) 3D reconstruction of duct at 14.5 dpc showing multi-cellular tdTomato+ cell patches containing K14+ (arrows) and K8+ cells (arrowheads). Scale bar, 50 μm.
Supplementary Figure 5 Activation of oncogenic Ras combined with Fbxw7 deletion in Lgr6-positive cells results in breast cancer.
(a) Description of the FRL mouse strain, derived from crossing three strains (Fbxw7f/f; LSL-KRasG12D; Lgr6-CreERT2). (b) Treatment scheme of 5-week-old FRL mice with tamoxifen and subsequent tumour analysis. (c) Immunohistochemistry of tumours from FRL mice 3 weeks post-injection with tamoxifen, stained with haematoxylin-eosin (HE) and probed for markers against basal (K5) and luminal (K8) cells. Scale bars, 100 μm. (d) Immunohistochemistry of tumours from FRL mice 8 weeks post-tamoxifen injection stained with HE and probed for K5, K8 and ERα. Scale bars, 100 μm. (e) Summary of BPL and FRL tumour lesions.
Supplementary Figure 6 Lgr6+ cells in MMTV-PyMT-expressing mammary glands.
(a) Mammary glands of Lgr6-CreERT2+/− (left) and Lgr6-CreERT2+/−:MMTV-PyMT+/− females (right) at P14. PyMT-induced lesions are found at P14 (inset). Scale bars, 50 μm, 20 μm (inset). (b) Mammary glands of Lgr6-CreERT2+/−(left) and Lgr6-CreERT2+/−:MMTV-PyMT+/− females (right) at P28. Middle T antigen-induced hyperplasia in puberty (right, inset). Scale bars, 100 μm, 20 μm (inset). (c) Middle T expression in early lesions of Lgr6-CreERT2+/−:MMTV-PyMT+/− mammary gland at P14. Lesions stained positive for PyMT (arrows) whereas normally appearing ducts were negative (arrowhead). Arrowheads in inset: PyMT− cells embedded within PyMT+ region. Scale bars, 100 μm, 20 μm (inset). (d) Middle T and K14 expression in pre-malignant mammary gland tissue of 2w-old (left panel) and mammary tumour of 15w-old (right) Lgr6-CreERT2+/−:MMTV-PyMT+/− females. Only rare Middle T oncogene expressing cells are positive for EGFP (arrows) and EGFP+ cells negative for PyMT oncogene are found (arrowheads). Scale bars, 50 μm (left), 200 μm (right panel), 50 μm (inset). (e) Adjacent EGFP+/tdTomato+ (yellow arrows/arrowheads) and tdTomato+ (red arrows/arrowheads) clones 2d post P28-induction. Green arrows/arrowheads depict EGFP+ cells. Scale bar, 50 μm. (f) GFP/EdU co-stained Lgr6-CreERT2+/−:MMTV-PyMT+/−mammary glands at 2w (left) and 15w of age (right). Left: EdU+ cells expressing Lgr6 (arrows). Scale bars, 50 μm. Right: Tumour regions with EdU+/Lgr6+ cells (arrows and inset). Scale bar, 200 μm, 25 μm (inset). (g) GFP immunostaining in mammary tumour of 24w-old PLT female induced at P29. Scale bars, 100 μm, 10 μm (inset). (h) Tumours from PLT females injected with sunflower-seed oil at 2w (left) or 4w (right) did not present with tdTomato+ cells. Scale bars, 1 mm. (i) Tamoxifen administration and time-points to analyse the contribution of Lgr6+ cells to tumour maintenance. (j) PLT mammary tumours 1d (inset) and 1w p.i. Scale bars, 100 μm (inset), 100 μm (inset). (k) Quantification of tdTomato+ area in PLT tumours after induction at 14w. (n = 6 tumours pooled from 4 mice) Line indicates mean. (l) Lgr6 in situ hybridization combined with immunostaining 1w after MPA (left) or MPA/DMBA (right) treatment demonstrating basal (arrows) and luminal (arrowheads) Lgr6+ cells in mammary glands undergoing chemical carcinogenesis. Scale bars, 25 μm.
Supplementary Figure 7 Lgr6+ cells contribute to tumour maintenance in the MMTV-PyMT model of breast carcinoma.
(a) Experimental setup to analyse chemotherapeutic resistance of Lgr6-expressing PyMT tumour cells in vitro. Tumour-bearing PLT mice were treated with tamoxifen and tumour organoids were cultured 24 h later. The organoids were treated with 2 μM doxorubicin for 48 h and allowed to recover until analysis. (b) Combined light microscopy and fluorescence images of tumour organoids comparing tdTomato tracing between untreated PLT organoids (upper panels) and doxorubicin-treated organoids (lower panels). tdTomato + cells survive chemotherapeutic treatment (lower middle panel) and are able to form completely traced, healthy organoids upon recovery (lower right panel). (c) Extent of tdTomato tracing within untreated and doxorubicin-treated organoids. (n = 8 pooled tumours from n = 4 mice, collected in 3 independent experiments). Mean ± s.e.m. ∗∗P < 0.01, ∗∗∗P < 0.001. (multiple unpaired t-tests). Scale bars, 200 Scale bars, 200 m, 100 μm (insets). (d) Scheme of PDL tumour cell isolation, allele recombination and DT treatment in vitro before colony formation analysis. Images show examples of tumour spheres from untreated (left) and DT-treated (right) cells. (e) mRNA expression for β-actin (Actb), Lgr6 and DTR (normalized over GAPDH) in tamoxifen- and DT-treated tumour cells from PDL mice. (n = 3 wells per group from n = 3 independent mice). Mean ± s.d. ∗∗∗P < 0.001 (unpaired two-tailed t-test) (f) Analysis of colony formation of primary PDL tumour cells after treatment with tamoxifen alone or tamoxifen plus diphtheria toxin. (n = 12 wells per group from n = 3 independent mice). Mean ± s.d. ∗P < 0.011 (Two-way ANOVA). (g) H&E staining of tumours formed by control and DT-treated PDL primary tumour cells injected subcutaneously into nude mice as shown in Fig. 7g. Scale bars, 500 μm (1x), 50 μm (40x). See Supplementary Table 2 for exact P values and source data for c,e,f.
Supplementary Figure 8 Scheme summarizing the contribution of Lgr6+ cells to postnatal mammary gland development, pregnancy, and luminal mammary tumours.
(a) During postnatal development, two populations of Lgr6+ mammary epithelial progenitor cells (basal and luminal) contribute to ductal morphogenesis. In adult virgin females, however, Lgr6 + cells do not actively add to tissue homeostasis and their number decreases over time. Their clonal potential is reactivated by pregnancy (and stimulation with ovarian hormones) and they contribute to the alveolar network over multiple pregnancies. (b) Lgr6+ progenitors can function as potent cells of origin of luminal mammary tumours Transformation of Lgr6+ progenitor cells with two different oncogenic combinations (loss of p53 and Brca1, or activation of mutant K-RasG12D and loss of Fbxw7, respectively) resulted in the formation of exclusively luminal mammary tumours, appearing with high penetrance. (c) Lgr6+ cells contribute to luminal, but not basal mammary tumours. Lineage tracing showed that luminal Lgr6+ progenitors contribute to MMTV-PyMT-induced tumours. In contrast, neither basal nor luminal Lgr6-expressing cells were traced in chemically induced, basaloid DMBA/MPA tumours.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1680 kb)
Supplementary Table 1
Supplementary Information (XLSX 49 kb)
Supplementary Table 2
Supplementary Information (XLSX 75 kb)
Rights and permissions
About this article
Cite this article
Blaas, L., Pucci, F., Messal, H. et al. Lgr6 labels a rare population of mammary gland progenitor cells that are able to originate luminal mammary tumours. Nat Cell Biol 18, 1346–1356 (2016). https://doi.org/10.1038/ncb3434
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3434
This article is cited by
-
LGR6 activates the Wnt/β-catenin signaling pathway and forms a β-catenin/TCF7L2/LGR6 feedback loop in LGR6high cervical cancer stem cells
Oncogene (2021)
-
Antigen retrieval and clearing for whole-organ immunofluorescence by FLASH
Nature Protocols (2021)
-
The role of R-spondin proteins in cancer biology
Oncogene (2021)
-
A Transgenic MMTV-Flippase Mouse Line for Molecular Engineering in Mammary Gland and Breast Cancer Mouse Models
Journal of Mammary Gland Biology and Neoplasia (2019)
-
G protein-coupled receptor LGR6 is an independent risk factor for colon adenocarcinoma
Frontiers of Medicine (2019)