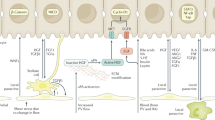
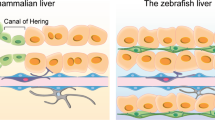
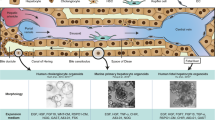
Abstract
Cell replacement in adult organs can be achieved through stem cell differentiation or the replication or transdifferentiation of existing cells. In the adult liver and pancreas, stem cells have been proposed to replace tissue cells, particularly following injury. Here we review how specialized cell types are produced in the adult liver and pancreas. Based on current evidence, we propose that the plasticity of differentiated cells, rather than stem cells, accounts for tissue repair in both organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Losick, V. P., Morris, L. X., Fox, D. T. & Spradling, A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 21, 159–171 (2011).
Donnelly, D. S., Zelterman, D., Sharkis, S. & Krause, D. S. Functional activity of murine CD34+ and CD34− hematopoietic stem cell populations. Exp. Hematol. 27, 788–796 (1999).
Krause, D. S. et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377 (2001).
Pevny, L. & Rao, M. S. The stem-cell menagerie. Trends Neurosci. 26, 351–359 (2003).
Kushner, J. A., Weir, G. C. & Bonner-Weir, S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab. 11, 2–3 (2010).
Criscimanna, A., Bertera, S., Esni, F., Trucco, M. & Bottino, R. in Type 1 Diabetes Complications (ed. P. D. Wagner) Ch. 17 (InTech, 2011).
Ziv, O., Glaser, B. & Dor, Y. The plastic pancreas. Dev. Cell 26, 3–7 (2013).
Grompe, M. Liver stem cells, where art thou? Cell Stem Cell 15, 257–258 (2014).
Yanger, K. & Stanger, B. Z. Facultative stem cells in liver and pancreas: fact and fancy. Dev. Dynam. 240, 521–529 (2011).
Duncan, A. W., Dorrell, C. & Grompe, M. Stem cells and liver regeneration. Gastroenterology 137, 466–481 (2009).
Winton, D. J., Blount, M. A. & Ponder, B. A. A clonal marker induced by mutation in mouse intestinal epithelium. Nature 333, 463–466 (1988).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Van der Flier, L. G. et al. The intestinal Wnt/TCF signature. Gastroenterology 132, 628–632 (2007).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Potten, C. S., Gandara, R., Mahida, Y. R., Loeffler, M. & Wright, N. A. The stem cells of small intestinal crypts: where are they? Cell Proliferat. 42, 731–750 (2009).
Potten, C. S. & Hendry, J. H. Differential regeneration of intestinal proliferative cells and cryptogenic cells after irradiation. Int. J. Radiat. Biol. Re. 27, 413–424 (1975).
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5+ cells dispensable. Nature 478, 255–259 (2011).
Buczacki, S. J. A. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 (2012).
Arias, I. M. & Boyer, J. L. The Liver: Biology and Pathobiology 3rd edn (Raven, 1994).
Sell, S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 33, 738–750 (2001).
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997).
Fausto, N. Liver regeneration. J. Hepatol. 32, 19–31 (2000).
Thorgeirsson, S. S. & Grisham, J. W. Overview of recent experimental studies on liver stem cells. Semin. Liver Dis. 23, 303–312 (2003).
Sell, S. & Ilic, Z. Liver Stem Cells (Landes Bioscience, 1997).
Sell, S. Liver stem cells. Mod. Pathol. 7, 105–112 (1994).
Miyajima, A., Tanaka, M. & Itoh, T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 14, 561–574 (2014).
Theise, N. D. et al. The canals of Hering and hepatic stem cells in humans. Hepatology 30, 1425–1433 (1999).
Zobiri, O., Deshayes, N. & Rathman-Josserand, M. Evolution of the clonogenic potential of human epidermal stem/progenitor cells with age. Stem Cells Cloning 5, 1–4 (2012).
Liu, J. M., Buchwald, M., Walsh, C. E. & Young, N. S. Fanconi anemia and novel strategies for therapy. Blood 84, 3995–4007 (1994).
Dorrell, C. et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 25, 1193–1203 (2011).
Shin, S. et al. Foxl1–Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 25, 1185–1192 (2011).
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Dorrell, C. et al. The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res. 13, 275–283 (2014).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Tarlow, B. D., Finegold, M. J. & Grompe, M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology 60, 278–289 (2014).
Arber, N., Zajicek, G. & Ariel, I. The streaming liver II: hepatocyte life history. Liver 8, 80–87 (1988).
Zajicek, G., Oren, R. & Weinreb, M., Jr. The streaming liver. Liver 5, 293–300 (1985).
Ponder, K. P. Analysis of liver development, regeneration, and carcinogenesis by genetic marking studies. FASEB J. 10, 673–682 (1996).
Bralet, M. P., Branchereau, S., Brechot, C. & Ferry, N. Cell lineage study in the liver using retroviral mediated gene transfer: evidence against the streaming of hepatocytes in normal liver. Amm J. Pathol. 144, 896–905 (1994).
Malato, Y. et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Invest. 121, 4850–4860 (2011).
Wang, B., Zhao, L., Fish, M., Logan, C. Y. & Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180–185 (2015).
Akhurst, B. et al. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 34, 519–522 (2001).
Paku, S., Schnur, J., Nagy, P. & Thorgeirsson, S. S. Origin and structural evolution of the early proliferating oval cells in rat liver. Am. J. Pathol. 158, 1313–1323 (2001).
Preisegger, K. H. et al. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab. Invest. 79, 103–109 (1999).
Roskams, T. & Desmet, V. Ductular reaction and its diagnostic significance. Semin. Diagn. Pathol. 15, 259–269 (1998).
Kiss, A., Schnur, J., Szabo, Z. & Nagy, P. Immunohistochemical analysis of atypical ductular reaction in the human liver, with special emphasis on the presence of growth factors and their receptors. Liver 21, 237–246 (2001).
Bucher, N. L. Experimental aspects of hepatic regeneration. New Engl. J. Med. 277, 738–746 (1967).
Frederiks, W. M., Marx, F., Chamuleau, R. A., van Noorden, C. J. & James, J. Immunocytochemical determination of ploidy class-dependent bromodeoxyuridine incorporation in rat liver parenchymal cells after partial hepatectomy. Histochemistry 93, 627–630 (1990).
Sakamoto, T. et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 29, 403–411 (1999).
Zaret, K. S. & Grompe, M. Generation and regeneration of cells of the liver and pancreas. Science 322, 1490–1494 (2008).
Carpentier, R. et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 141, 1432–1438 (2011).
Kaestner, K. H. The making of the liver: developmental competence in foregut endoderm and induction of the hepatogenic program. Cell Cycle 4, 1146–1148 (2005).
Hixson, D. C., Affigne, S., Faris, R. A. & McBride, A. C. Delineation of antigenic pathways of ethionine-induced liver cancer in the rat. Carcinogenesis 18, 1169–1175 (1997).
Petersen, B. E., Goff, J. P., Greenberger, J. S. & Michalopoulos, G. K. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology 27, 433–445 (1998).
Evarts, R. P., Nagy, P., Marsden, E. & Thorgeirsson, S. S. A precursor–product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 8, 1737–1740 (1987).
Evarts, R. P., Nagy, P., Nakatsukasa, H., Marsden, E. & Thorgeirsson, S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 49, 1541–1547 (1989).
Lazaro, C. A., Rhim, J. A., Yamada, Y. & Fausto, N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 58, 5514–5522 (1998).
Wang, X. et al. The origin and liver repopulating capacity of murine oval cells. Proc. Natl Acad. Sci. USA 100, 11881–11888 (2003).
Petersen, B. E. et al. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology 37, 632–640 (2003).
Espanol-Suner, R. et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143, 1564–1575 (2012).
Furuyama, K. et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 (2011).
Yanger, K. et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 15, 340–349 (2014).
Schaub, J. R., Malato, Y., Gormond, C. & Willenbring, H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 8, 933–939 (2014).
Shin, S., Upadhyay, N., Greenbaum, L. E. & Kaestner, K. H. Ablation of Foxl1–Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology 148, 192–202 (2015).
Lu, W. Y. et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 17, 971–983 (2015).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Bioulac-Sage, P. & Balabaud, C. Human cirrhosis: monoclonal regenerative nodules derived from hepatic progenitor cells abutting ductular reaction. Gastroen. Clin. Biol. 34, 267–269 (2010).
Richardson, M. M. et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133, 80–90 (2007).
Clouston, A. D. et al. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 41, 809–818 (2005).
Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013).
Sekiya, S. & Suzuki, A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am. J. Pathol. 184, 1468–1478 (2014).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Fan, B. et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Invest. 122, 2911–2915 (2012).
Sekiya, S. & Suzuki, A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J. Clin. Invest. 122, 3914–3918 (2012).
Font-Burgada, J. et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779 (2015).
Githens, S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J. Pediat. Gastr. Nutr. 7, 486–506 (1988).
Sangiorgi, E. & Capecchi, M. R. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc. Natl Acad. Sci. USA 106, 7101–7106 (2009).
Teta, M., Long, S. Y., Wartschow, L. M., Rankin, M. M. & Kushner, J. A. Very slow turnover of β-cells in aged adult mice. Diabetes 54, 2557–2567 (2005).
Hayashi, K. Y. et al. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch. Histol. Cytol. 66, 163–174 (2003).
Hayashi, K., Takahashi, T., Kakita, A. & Yamashina, S. Regional differences in the cellular proliferation activity of the regenerating rat pancreas after partial pancreatectomy. Arch. Histol. Cytol. 62, 337–346 (1999).
Elsasser, H. P., Adler, G. & Kern, H. F. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas 1, 421–429 (1986).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).
Nir, T., Melton, D. A. & Dor, Y. Recovery from diabetes in mice by β-cell regeneration. J. Clin. Invest. 117, 2553–2561 (2007).
Menge, B. A. et al. Long-term recovery of β-cell function after partial pancreatectomy in humans. Metabolism 61, 620–624 (2012).
Menge, B. A. et al. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 57, 142–149 (2008).
Phillip, V. et al. Volumetric gain of the human pancreas after left partial pancreatic resection: a CT-scan based retrospective study. Pancreatology 15, 542–547 (2015).
Bonner-Weir, S., Trent, D. F. & Weir, G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J. Clin. Invest. 71, 1544–1553 (1983).
Brockenbrough, J. S., Weir, G. C. & Bonner-Weir, S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37, 232–236 (1988).
Bouwens, L. & Pipeleers, D. G. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 41, 629–633 (1998).
Wang, T. C. et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor α-induced ductular precursor cells. J. Clin. Invest. 92, 1349–1356 (1993).
Wang, R. N., Klöppel, G. & Bouwens, L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38, 1405–1411 (1995).
Xu, X. et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008).
Yamamoto, K. et al. Recombinant human β-cellulin promotes the neogenesis of β-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes 49, 2021–2027 (2000).
Tokui, Y. et al. Neogenesis and proliferation of β-cells induced by human betacellulin gene transduction via retrograde pancreatic duct injection of an adenovirus vector. Biochem. Biophys. Res. Commun. 350, 987–993 (2006).
Peshavaria, M. et al. Regulation of pancreatic β-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 55, 3289–3298 (2006).
Gu, G., Brown, J. R. & Melton, D. A. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech. Dev. 120, 35–43 (2003).
Kopp, J. L. et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665 (2011).
Solar, M. et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 (2009).
Kopinke, D. & Murtaugh, L. C. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev. Biol. 10, 38 (2010).
Seaberg, R. M. et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 22, 1115–1124 (2004).
Smukler, S. R. et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8, 281–293 (2011).
Zulewski, H. et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50, 521–533 (2001).
Suzuki, A., Nakauchi, H. & Taniguchi, H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 53, 2143–2152 (2004).
Gao, R. et al. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52, 2007–2015 (2003).
Ramiya, V. K. et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 6, 278–282 (2000).
Jin, L. et al. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc. Natl Acad. Sci. USA 110, 3907–3912 (2013).
Lee, J. et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2, e00940 (2013).
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013).
Rovira, M. et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl Acad. Sci. USA 107, 75–80 (2010).
Bensley, R. R. Studies on the pancreas of the guinea pig. Am. J. Anat. 12, 297–388 (1911).
Dor, Y., Brown, J., Martinez, O. I. & Melton, D. A. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Desai, B. M. et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 117, 971–977 (2007).
Strobel, O. et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133, 1999–2009 (2007).
Li, W.-C. et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J. Cell Sci. 123, 2792–2802 (2010).
Sharma, A. et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48, 507–513 (1999).
Jensen, J. N. et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. YGAST 128, 728–741 (2005).
Bonner-Weir, S. et al. Beta-cell growth and regeneration: replication is only part of the story. Diabetes 59, 2340–2348 (2010).
Criscimanna, A. et al. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology 141, 1451–1462 (2011).
Van de Casteele, M. et al. Neurogenin3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 4, e523 (2013).
Xiao, X. et al. No evidence for β cell neogenesis in murine adult pancreas. J. Clin. Invest. 123, 2207–2217 (2013).
Van De Casteele, M. et al. Partial duct ligation: β-cell proliferation and beyond. Diabetes 63, 2567–2577 (2014).
Pan, F. C. et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140, 751–764 (2013).
Jonckheere, N. et al. Analysis of mPygo2 mutant mice suggests a requirement for mesenchymal Wnt signaling in pancreatic growth and differentiation. Dev. Biol. 318, 224–235 (2008).
Murtaugh, L. C., Law, A. C., Dor, Y. & Melton, D. A. Beta-catenin is essential for pancreatic acinar but not islet development. Development 132, 4663–4674 (2005).
Papadopoulou, S. & Edlund, H. Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes 54, 2844–2851 (2005).
Dessimoz, J., Bonnard, C., Huelsken, J. & Grapin-Botton, A. Pancreas-specific deletion of β-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr. Biol. 15, 1677–1683 (2005).
Rankin, M. M. et al. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 62, 1634–1645 (2013).
Blaine, S. A. et al. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 137, 2289–2296 (2010).
Inada, A. et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl Acad. Sci. USA 105, 19915–19919 (2008).
Kopinke, D. et al. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431–441 (2011).
Kopp, J. L. et al. Progenitor cell domains in the developing and adult pancreas. Cell Cycle 10, 1921–1927 (2011).
Strobel, O. et al. Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc. Natl Acad. Sci. USA 104, 4419–4424 (2007).
Chera, S. et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514, 503–507 (2014).
Hosokawa, S. et al. Impact of Sox9 dosage and Hes1-mediated Notch signaling in controlling the plasticity of adult pancreatic duct cells in mice. Sci. Rep. 5, 8518 (2015).
Bockman, D. E., Boydston, W. R. & Anderson, M. C. Origin of tubular complexes in human chronic pancreatitis. Am. J. Surgery 144, 243–249 (1982).
Brereton, M. F. et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 5, 4639 (2014).
Talchai, C., Xuan, S., Lin, H. V., Sussel, L. & Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012).
Morris, J. P., Cano, D. A., Sekine, S., Wang, S. C. & Hebrok, M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 120, 508–520 (2010).
Kopp, J. L. et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750 (2012).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Acknowledgements
We thank A. C. Carrano (Univ. California, USA) for help with writing the manuscript and constructive comments. We apologize to our colleagues whose references were omitted owing to space constraints. J.L.K. is supported by grants from the Juvenile Diabetes Research Foundation (JDRF), National Pancreatic Cancer Canada Foundation, Pancreas Centre British Columbia, Canadian Institutes of Health Research and the National Institutes of Health. M.G. is supported by grants from the National Institutes of Health (DK05192 and DK104143) and the Helmsley Charitable Trust. M.S. is supported by grants from the National Institutes of Health (DK078803 and DK068471), the JDRF, the Helmsley Charitable Trust and the California Institute for Regenerative Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kopp, J., Grompe, M. & Sander, M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol 18, 238–245 (2016). https://doi.org/10.1038/ncb3309
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3309
This article is cited by
-
Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: a comprehensive and systematic review
Signal Transduction and Targeted Therapy (2023)
-
Significance of CCNs in liver regeneration
Journal of Cell Communication and Signaling (2023)
-
Liver organoids in domestic animals: an expected promise for metabolic studies
Veterinary Research (2021)
-
The immunological and metabolic landscape in primary and metastatic liver cancer
Nature Reviews Cancer (2021)
-
The evolving definition of salivary gland stem cells
npj Regenerative Medicine (2021)