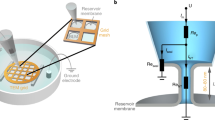
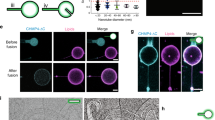
Abstract
Dynamin, the paradigmatic membrane fission catalyst, assembles as helical scaffolds that hydrolyse GTP to sever the tubular necks of clathrin-coated pits. Using a facile assay system of supported membrane tubes (SMrT) engineered to mimic the dimensions of necks of clathrin-coated pits, we monitor the dynamics of a dynamin-catalysed tube-severing reaction in real time using fluorescence microscopy. We find that GTP hydrolysis by an intact helical scaffold causes progressive constriction of the underlying membrane tube. On reaching a critical dimension of 7.3 nm in radius, the tube undergoes scission and concomitant splitting of the scaffold. In a constant GTP turnover scenario, scaffold assembly and GTP hydrolysis-induced tube constriction are kinetically inseparable events leading to tube-severing reactions occurring at timescales similar to the characteristic fission times seen in vivo. We anticipate SMrT templates to allow dynamic fluorescence-based detection of conformational changes occurring in self-assembling proteins that remodel membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Faelber, K. et al. Structural insights into dynamin-mediated membrane fission. Structure 20, 1621–1628 (2012).
Schmid, S. L. & Frolov, V. A. Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 27, 79–105 (2011).
Ferguson, S. M. & De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75–88 (2012).
Chappie, J. S. & Dyda, F. Building a fission machine–structural insights into dynamin assembly and activation. J. Cell Sci. 2773–2784 (2013).
Chappie, J. S., Acharya, S., Leonard, M., Schmid, S. L. & Dyda, F. G domain dimerization controls dynamin’s assembly-stimulated GTPase activity. Nature 465, 435–440 (2010).
Kozlovsky, Y. & Kozlov, M. M. Membrane fission: model for intermediate structures. Biophys. J. 85, 85–96 (2003).
Frolov, V. A., Escalada, A., Akimov, S. A. & Shnyrova, A. V. Geometry of membrane fission. Chem. Phys. Lipids 185, 129–140 (2015).
Zhang, P. & Hinshaw, J. E. Three-dimensional reconstruction of dynamin in the constricted state. Nat. Cell Biol. 3, 922–926 (2001).
Roux, A. et al. Membrane curvature controls dynamin polymerization. Proc. Natl Acad. Sci. USA 107, 4141–4146 (2010).
Bashkirov, P. V. et al. GTPase cycle of Dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 135, 1276–1286 (2008).
Shnyrova, A. V. et al. Geometric catalysis of membrane fission driven by flexible dynamin rings. Science 339, 1433–1436 (2013).
Morlot, S. et al. Membrane shape at the edge of the Dynamin Helix sets location and duration of the fission reaction. Cell 151, 619–629 (2012).
Chappie, J. S. et al. A pseudoatomic model of the Dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell 147, 209–222 (2011).
Sundborger, A. C. et al. A dynamin mutant defines a superconstricted prefission state. Cell Rep. 8, 734–742 (2014).
Roux, A. Reaching a consensus on the mechanism of dynamin? F1000Prime Rep. 6, 86 (2014).
Iversen, T. G., Skretting, G., van Deurs, B. & Sandvig, K. Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc. Natl Acad. Sci. USA 100, 5175–5180 (2003).
Hsieh, W.-T. et al. Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir 28, 12838–12843 (2012).
Jung, H., Robison, A. D. & Cremer, P. S. Detecting protein-ligand binding on supported bilayers by local pH modulation. J. Am. Chem. Soc. 131, 1006–1014 (2009).
Pucadyil, T. J. & Schmid, S. L. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135, 1263–1275 (2008).
Ramachandran, R. et al. Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission. Mol. Biol. Cell 20, 4630–4639 (2009).
Roux, A., Uyhazi, K., Frost, A. & De Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 (2006).
Sweitzer, S. M. & Hinshaw, J. E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93, 1021–1029 (1998).
Danino, D., Moon, K.-H. & Hinshaw, J. E. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J. Struct. Biol. 147, 259–267 (2004).
Doyon, J. B. et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat. Cell Biol. 13, 331–337 (2011).
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Cocucci, E., Gaudin, R. & Kirchhausen, T. Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol. Biol. Cell 25, 3595–3609 (2014).
Mattila, J.-P. et al. A hemi-fission intermediate links two mechanistically distinct stages of membrane fission. Nature 524, 109–113 (2015).
Lemmon, M. A. & Ferguson, K. M. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350, 1–8 (2000).
Vallis, Y., Wigge, P., Marks, B., Evans, P. R. & McMahon, H. T. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr. Biol. 9, 257–260 (1999).
Mehrotra, N., Nichols, J. & Ramachandran, R. Alternate pleckstrin homology domain orientations regulate dynamin-catalyzed membrane fission. Mol. Biol. Cell 25, 879–890 (2014).
Fuhrmans, M. & Müller, M. Coarse-grained simulation of dynamin-mediated fission. Soft Matter 11, 1464–1480 (2015).
Mears, J. A., Ray, P. & Hinshaw, J. E. A corkscrew model for dynamin constriction. Structure 15, 1190–1202 (2007).
Turner, D. K. et al. Reduction of artifacts in fluorescence correlation spectroscopy due to sample adsorption on optical glass surfaces. Appl. Spectrosc. 67, 692–698 (2013).
Neumann, S., Pucadyil, T. J. & Schmid, S. L. Analyzing membrane remodeling and fission using supported bilayers with excess membrane reservoir. Nat. Protoc. 8, 213–222 (2013).
Ellenberg, J. et al. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 138, 1193–1206 (1997).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Kunding, A. H., Mortensen, M. W., Christensen, S. M. & Stamou, D. A fluorescence-based technique to construct size distributions from single-object measurements: application to the extrusion of lipid vesicles. Biophys. J. 95, 1176–1188 (2008).
Shlomovitz, R., Gov, N. S. & Roux, A. Membrane-mediated interactions and the dynamics of dynamin oligomers on membrane tubes. New J. Phys. 13, 065008 (2011).
Acknowledgements
We thank S. Schmid, R. Mallik and the Pucadyil laboratory members for discussions and critical comments on the manuscript, S. Holkar for the scanning electron microscopy, and V. Vitthal for confocal microscopy. S.D. and S.C.K. acknowledge the Council for Scientific and Industrial Research (CSIR) for fellowships. T.J.P. is an Intermediate Fellow of the Wellcome Trust-DBT India Alliance and thanks the Alliance and IISER Pune for funds.
Author information
Authors and Affiliations
Contributions
S.D. and T.J.P. designed experiments. S.D. performed all experiments. S.D. and S.C.K. designed and standardized preparation of SMrT templates. S.D. and T.J.P analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Calculation of calibration constant to equate tube fluorescence to physical dimensions.
Fluorescence micrographs of SMrT templates with preassembled scaffolds are acquired in the tube fluorescence channel (Step 1). The bimodal frequency distribution of pixel intensities along the tube length is fitted to a sum of 2 gaussian function (Step 2). The lower mean represents the tube fluorescence under the scaffold, which is then equated to the tube radius by I = K∗R2 where, I = tube fluorescence under scaffold; R = tube radius, 11.2 nm9 to calculate the calibration constant K.
Supplementary Figure 2 GTP hydrolysis-induced tube scission leads to splitting of scaffolds.
A panel of kymographs from time-lapse movies monitoring GTP addition to Alexa488-labeled dynamin scaffolds preassembled on SMrT templates.
Supplementary information
Supplementary Information
Supplementary Information (PDF 799 kb)
Dynamics of dynamin scaffold assembly on SMrT templates.
The movie shows tube fluorescence changes on scaffold assembly on SMrT templates. Scale bar = 5 μm. (MOV 4242 kb)
Dynamics of dynamin scaffold assembly on a freestanding membrane tether.
The movie shows tube fluorescence changes on scaffold assembly on a freestanding tether. Scale bar = 5 μm. (MOV 1716 kb)
Tube scission events with preassembed scaffolds on SMrT templates.
The movie shows tube fluorescence changes in response to GTP addition to preassembled scaffolds on SMrT templates. Scale bar = 5 μm. (MOV 501 kb)
Tube scission events with dynamin in the constant presence of GTP on SMrT templates.
The movie shows tube fluorescence changes in response to dynamin addition to SMrT templates bathed in excess (1 mM) GTP. Scale bar = 5 μm. (MOV 1237 kb)
Rights and permissions
About this article
Cite this article
Dar, S., Kamerkar, S. & Pucadyil, T. A high-throughput platform for real-time analysis of membrane fission reactions reveals dynamin function. Nat Cell Biol 17, 1588–1596 (2015). https://doi.org/10.1038/ncb3254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3254
This article is cited by
-
The HEAT repeat protein HPO-27 is a lysosome fission factor
Nature (2024)
-
Metal-Binding Propensity in the Mitochondrial Dynamin-Related Protein 1
The Journal of Membrane Biology (2022)
-
Function and regulation of the divisome for mitochondrial fission
Nature (2021)
-
Reconstitution and real-time quantification of membrane remodeling by single proteins and protein complexes
Nature Protocols (2020)
-
Dynamic constriction and fission of endoplasmic reticulum membranes by reticulon
Nature Communications (2019)