Abstract
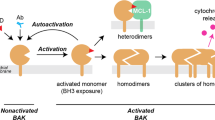
Multidomain pro-apoptotic BAX and BAK, once activated, permeabilize mitochondria to trigger apoptosis, whereas anti-apoptotic BCL-2 members preserve mitochondrial integrity. The BH3-only molecules (BH3s) promote apoptosis by either activating BAX–BAK or inactivating anti-apoptotic members. Here, we present biochemical and genetic evidence that NOXA is a bona fide activator BH3. Using combinatorial gain-of-function and loss-of-function approaches in Bid−/−Bim−/−Puma−/−Noxa−/− and Bax−/−Bak−/− cells, we have constructed an interconnected hierarchical model that accommodates and explains how the intricate interplays between the BCL-2 members dictate cellular survival versus death. BID, BIM, PUMA and NOXA directly induce stepwise, bimodal activation of BAX–BAK. BCL-2, BCL-XL and MCL-1 inhibit both modes of BAX–BAK activation by sequestering activator BH3s and ‘BH3-exposed’ monomers of BAX–BAK, respectively. Furthermore, autoactivation of BAX and BAK can occur independently of activator BH3s through downregulation of BCL-2, BCL-XL and MCL-1. Our studies lay a foundation for targeting the BCL-2 family for treating diseases with dysregulated apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gross, A., McDonnell, J. M. & Korsmeyer, S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 (2008).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001).
Newmeyer, D. D. & Ferguson-Miller, S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490 (2003).
Kim, H. et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 8, 1348–1358 (2006).
Westphal, D., Kluck, R. M. & Dewson, G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 21, 196–205 (2014).
Wei, M. C. et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060–2071 (2000).
Cheng, E. H. et al. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8, 705–711 (2001).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183–192 (2002).
Chen, L. et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005).
Kuwana, T. et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525–535 (2005).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006).
Kim, H. et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36, 487–499 (2009).
Ren, D. et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330, 1390–1393 (2010).
Sattler, M. et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997).
Czabotar, P. E. et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc. Natl Acad. Sci. USA 104, 6217–6222 (2007).
Czabotar, P. E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 (2013).
Moldoveanu, T. et al. BID-induced structural changes in BAK promote apoptosis. Nat. Struct. Mol. Biol. 20, 589–597 (2013).
Leshchiner, E. S., Braun, C. R., Bird, G. H. & Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proc. Natl Acad. Sci. USA 110, E986–E995 (2013).
Edwards, A. L. et al. Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chem. Biol. 20, 888–902 (2013).
Brouwer, J. M. et al. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol. Cell 55, 938–946 (2014).
Dewson, G. et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3: groove interactions. Mol. Cell 30, 369–380 (2008).
Gavathiotis, E., Reyna, D. E., Davis, M. L., Bird, G. H. & Walensky, L. D. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell 40, 481–492 (2010).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Desagher, S. et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144, 891–901 (1999).
Willis, S. N. et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859 (2007).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Martinou, J. C. & Youle, R. J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 (2011).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003).
Kuwana, T. et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 (2002).
Lovell, J. F. et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074–1084 (2008).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Shibue, T. et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 17, 2233–2238 (2003).
Naik, E., Michalak, E. M., Villunger, A., Adams, J. M. & Strasser, A. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J. Cell Biol. 176, 415–424 (2007).
Lowe, S. W., Ruley, H. E., Jacks, T. & Housman, D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74, 957–967 (1993).
Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J. & Korsmeyer, S. J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 (2003).
Willis, S. N. et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294–1305 (2005).
Tu, H. C. et al. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc. Natl Acad. Sci. USA 106, 1093–1098 (2009).
Cheng, E. H., Levine, B., Boise, L. H., Thompson, C. B. & Hardwick, J. M. Bax-independent inhibition of apoptosis by Bcl-XL . Nature 379, 554–556 (1996).
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Westphal, D., Dewson, G., Czabotar, P. E. & Kluck, R. M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 1813, 521–531 (2011).
Edlich, F. et al. Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 (2011).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Fu, N. Y., Sukumaran, S. K., Kerk, S. Y. & Yu, V. C. Baxbeta: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol. Cell 33, 15–29 (2009).
Du, H. et al. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J. Biol. Chem. 286, 491–501 (2011).
Dai, H. et al. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J. Cell Biol. 194, 39–48 (2011).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Davids, M. S. & Letai, A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol. 30, 3127–3135 (2012).
Walensky, L. D. From mitochondrial biology to magic bullet: navitoclax disarms BCL-2 in chronic lymphocytic leukemia. J. Clin. Oncol. 30, 554–557 (2012).
Anderson, M. A., Huang, D. & Roberts, A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin. Hematol. 51, 219–227 (2014).
Takeuchi, O. et al. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc. Natl Acad. Sci. USA 102, 11272–11277 (2005).
Takeda, S. et al. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev. 20, 2397–2409 (2006).
Yethon, J. A., Epand, R. F., Leber, B., Epand, R. M. & Andrews, D. W. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 278, 48935–48941 (2003).
Acknowledgements
We apologize to all of the investigators whose research could not be appropriately cited owing to space limitation. We thank H.-F. Chen and S. Han for technical assistance. This work was supported by grants to E.H.C. from the NIH (R01CA125562) and the American Cancer Society (118518-RSG-10-030-01-CCG), and to E.G. from the NIH (R01CA178394) This work was also supported by the NIH P30CA008748.
Author information
Authors and Affiliations
Contributions
H.-C.C. designed and conducted experiments, and analysed data. E.H.C. designed research, analysed data and supervised the project. H.-C.T., M.K., Y.H., H.K., A.I.-Y., Y.T.G. and D.E.R. conducted experiments. J.J.H. and E.G. supervised some experiments. H.C.T., D.R., P.M.C., S.T. and C.-P.L. generated essential reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 Triple deletion of Bid, Bim, and Puma fails to completely block intrinsic apoptosis in mouse embryonic fibroblasts.
E1A/Ras-transformed wild-type (WT), Bid−/−Bim−/−, Bim−/−Puma−/−, or Bid−/−Bim−/−Puma−/− MEFs were untreated, or cultured in the absence of serum or glucose, or in the presence of tunicamycin, thapsigargin or etoposide. Cell death was quantified by annexin-V staining at the indicated times (mean ± s.d., n = 3 independent experiments). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (Student’s t-test).
Supplementary Figure 3
(a) Reticulocyte lysates abrogates the cytochrome c releasing activity of NOXA. Isolated wild-type mitochondria were incubated with IVTT mouse NOXA generated using wheat germ extract (WGE) in the absence or presence of reticulocyte lysates (R) or IVTT mouse NOXA generated using reticulocyte lysates (R) in the absence or presence of wheat germ extract (WGE) at 30 °C for 30 min, after which the release of cytochrome c was quantified by ELISA assays (mean ± s.d., n = 3 independent experiments). (b) Isolated wild-type mitochondria were incubated with IVTT mouse tBID generated using wheat germ extract (WGE) in the absence or presence of reticulocyte lysates (R) at 30 °C for 30 min, after which the release of cytochrome c was quantified by ELISA assays (mean ± s.d., n = 3 independent experiments). (c) The expression of Noxa mRNA is comparable in wild-type and Bid−/−Bim−/−Puma−/− MEFs. The mRNA levels of Noxa in primary wild-type or Bid−/−Bim−/−Puma−/− MEFs were assessed by qRT-PCR. Data were normalized against GAPDH (mean ± s.d., n = 3 independent experiments). (d) Bid−/−Bim−/−Puma−/− TKO and Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice display persistence of interdigital webs. Ventral views of paws from WT, Bid−/−Bim−/−Puma−/− TKO, and Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice at 6 to 8 weeks of age. Representative images from at least 20 mice are shown. (e) Bid−/−Bim−/−Puma−/− TKO and Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice fail to develop external vaginal introituses. Photographs of vaginal openings from WT, Bid−/−Bim−/−Puma−/− TKO, and Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice at 6 to 8 weeks of age. Arrows point to external vaginal region. Representative images from at least 6 mice are shown. (f) Noxa expression is lower both at the basal level and in response to genotoxic stress in T-cells than transformed mouse embryonic fibroblasts. The mRNA levels of Noxa in the indicated cells untreated or treated with etoposide for 6 h were assessed by qRT-PCR. Data were normalized against 18S rRNA (mean ± s.d., n = 3 independent experiments). ∗∗, P < 0.01 (Student’s t-test).
Supplementary Figure 4 Total body irradiation-induced apoptosis in spleen was greatly reduced in both Bid−/−Bim−/−Puma−/− and Bid−/−Bim−/−Puma−/−Noxa−/− mice.
(a) Apoptosis in the spleens of wild-type, Bid−/−Bim−/−Puma−/− TKO, or Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice at 8 to 17 weeks of age at 4 h after 18 Gy total body irradiation was assessed by TUNEL staining. Representative light microscopy images from two independent experiments are shown (brown, magnification 200×). Scale bars, 50 μm. (b) Wild-type, Bid−/−Bim−/−Puma−/− TKO, or Bid−/−Bim−/−Puma−/−Noxa−/− QKO mice at 8 to 10 weeks of age were unirradiated (n = 3 for each genotype) or irradiated with 5 Gy total body irradiation (n = 3 for each genotype). 3 days later, total numbers of splenocytes and CD4+ splenocytes were assessed. The percentage of survival was defined as the numbers of viable cells from irradiated mice divided by those from unirradiated ones with the same genotypes (mean ± s.d., n = 3 for each genotype). ∗∗, P < 0.01 (Student’s t-test).
Supplementary Figure 5
(a) Immunoblot analyses of siRNA- or shRNA-mediated knockdown. SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− MEFs infected with retrovirus expressing shRNA against the indicated genes, or transfected with the indicated siRNA, were harvested at 72 h later and analyzed with immunoblots using the indicated antibodies. SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− MEFs were sequentially infected with retrovirus expressing HA-tagged BIK or BMF and retrovirus expressing shRNA against luciferase, Bik, or Bmf. The expression of HA-tagged BIK or BMF was assessed by an anti-HA immunoblot. (b) SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− MEFs infected with retrovirus expressing shRNA again luciferase, Bmf, or Bik, were harvested at 72 h later. The mRNA levels of Bmf or Bik were assessed by qRT-PCR. Data were normalized against GAPDH (mean ± s.d., n = 3 independent experiments). (c) SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− MEFs transfected with scrambled siRNA (siScr) or siRNA against Hrk were harvested at 72 h later. The mRNA levels of Hrk were assessed by qRT-PCR. Data were normalized against GAPDH (mean ± s.d., n = 3 independent experiments). (d) SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− MEFs transfected with scrambled siRNA (siScr) or siRNA against Hrk were untreated or treated with etoposide for 2 days. Cell death was quantified by annexin-V staining (mean ± s.d., n = 3 independent experiments). ∗∗, P < 0.01; ∗∗∗, P < 0.001 (Student’s t-test).
Supplementary Figure 6
(a) SV40-transformed wild-type MEFs, untreated or treated with etoposide, tunicamycin (TC), or thapsigargin (TG), were subjected to immunoblot analysis using the indicated antibodies. (b) CD4+ T cells purified from the spleens of two wild-type mice were untreated or treated with etoposide for 18 h, and subjected to immunoblot analysis using the indicated antibodies. (c) SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− QKO MEFs, untreated or treated with etoposide, or irradiated with 20 Gy γ-irradiation or UV-C, were subjected to immunoblot analysis using the indicated antibodies. (d) SV40-transformed wild-type, Bid−/−Bim−/−Puma−/− TKO, Bid−/−Bim−/−Puma−/−Noxa−/− QKO, or Bax−/−Bak−/− DKO MEFs were untreated or irradiated with 20 Gy γ-irradiation. Cell death was quantified by annexin-V staining at the indicated times (mean ± s.d., n = 3 independent experiments). (e) Knockdown of Bcl-2, Bcl-xL or Mcl-1 does not sensitize QKO cells to overexpression of BAD, BMF, BIK or HRK. SV40-transformed Bid−/−Bim−/−Puma−/−Noxa−/− QKO MEFs were transfected with scrambled siRNA (siScr) or siRNA against Bcl-2, Bcl-xL or Mcl-1. After 2 days, cells were infected with retrovirus expressing GFP or the indicated BH3-only proteins. Cell death was quantified by annexin-V staining at 30 h (mean ± s.d., n = 3 independent experiments). (f) SV40-transformed wild-type or Bid−/−Bim−/−Puma−/− TKO MEFs stably expressing GFP, HA-BCL-2, HA-BCL-XL or HA-MCL-1 were subjected to anti-HA immunoprecipitation in 0.2% NP-40 lysis buffer. The input (5%) and immunoprecipitates were analyzed by anti-BAX, anti-BAK, and anti-HA immunoblots. ∗∗, P < 0.01; ∗∗∗, P < 0.001 (Student’s t-test).
Supplementary Figure 8 Full scans of immunoblots.
In some experiments, membranes were cut prior to probing each strip with a separate antibody.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1660 kb)
Rights and permissions
About this article
Cite this article
Chen, HC., Kanai, M., Inoue-Yamauchi, A. et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat Cell Biol 17, 1270–1281 (2015). https://doi.org/10.1038/ncb3236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3236
This article is cited by
-
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
-
Targeting PRSS23 with tipranavir induces gastric cancer stem cell apoptosis and inhibits growth of gastric cancer via the MKK3/p38 MAPK-IL24 pathway
Acta Pharmacologica Sinica (2024)
-
A novel inhibitory BAK antibody enables assessment of non-activated BAK in cancer cells
Cell Death & Differentiation (2024)
-
Novel meriolin derivatives activate the mitochondrial apoptosis pathway in the presence of antiapoptotic Bcl-2
Cell Death Discovery (2024)
-
Silica nanoparticles induce male reproductive toxicity via Crem hypermethylation mediated spermatocyte apoptosis and sperm flagella damage
Environmental Science and Pollution Research (2024)