Abstract
Senescent cells secrete a combination of factors collectively known as the senescence-associated secretory phenotype (SASP). The SASP reinforces senescence and activates an immune surveillance response, but it can also show pro-tumorigenic properties and contribute to age-related pathologies. In a drug screen to find new SASP regulators, we uncovered the mTOR inhibitor rapamycin as a potent SASP suppressor. Here we report a mechanism by which mTOR controls the SASP by differentially regulating the translation of the MK2 (also known as MAPKAPK2) kinase through 4EBP1. In turn, MAPKAPK2 phosphorylates the RNA-binding protein ZFP36L1 during senescence, inhibiting its ability to degrade the transcripts of numerous SASP components. Consequently, mTOR inhibition or constitutive activation of ZFP36L1 impairs the non-cell-autonomous effects of senescent cells in both tumour-suppressive and tumour-promoting contexts. Altogether, our results place regulation of the SASP as a key mechanism by which mTOR could influence cancer, age-related diseases and immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
28 August 2015
In the original version of this Article, a sentence was changed in error during production. This sentence should have read ‘To understand to what extent mTOR regulates the SASP, we analysed the secretome of senescent cells by mass spectrometry (MS; refs 24, 25).’ There was a further typographical error in one instance of ‘SASP components’. These errors have been corrected in all online versions of the Article.
References
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Salama, R., Sadaie, M., Hoare, M. & Narita, M. Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kuilman, T. & Peeper, D. S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 9, 81–94 (2009).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Hoenicke, L. & Zender, L. Immune surveillance of senescent cells–biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33, 1123–1126 (2012).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Jing, H. et al. Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev. 25, 2137–2146 (2011).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Freund, A., Patil, C. K. & Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30, 1536–1548 (2011).
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Ruvinsky, I. & Meyuhas, O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31, 342–348 (2006).
Richter, J. D. & Sonenberg, N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433, 477–480 (2005).
Thoreen, C. C. et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 (2012).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Iglesias-Bartolome, R. et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414 (2012).
Dorr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Acosta, J. C., Snijders, A. P. & Gil, J. Unbiased characterization of the senescence-associated secretome using SILAC-based quantitative proteomics. Methods Mol. Biol. 965, 175–184 (2013).
Cutillas, P. R. & Vanhaesebroeck, B. Quantitative profile of five murine core proteomes using label-free functional proteomics. Mol. Cell. Proteomics 6, 1560–1573 (2007).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Pospelova, T. V. et al. Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle 11, 2402–2407 (2012).
Demidenko, Z. N. et al. Rapamycin decelerates cellular senescence. Cell Cycle 8, 1888–1895 (2009).
Bundscherer, A., Vogt, T., Kohl, G., Landthaler, M. & Hafner, C. Antiproliferative effects of rapamycin and celecoxib in angiosarcoma cell lines. Anticancer Res. 30, 4017–4023 (2010).
Dowling, R. J. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 (2010).
Dieterich, D. C., Link, A. J., Graumann, J., Tirrell, D. A. & Schuman, E. M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl Acad. Sci. USA 103, 9482–9487 (2006).
Casado, P. et al. Kinase-substrate enrichment analysis provides insights into the heterogeneity of signaling pathway activation in leukemia cells. Sci. Signal. 6, rs6 (2013).
Gaestel, M. MAPKAP kinases—MKs—two’s company, three’s a crowd. Nat. Rev. Mol. Cell Biol. 7, 120–130 (2006).
Hsieh, A. C. et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012).
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009).
Ross, C. R., Brennan-Laun, S. E. & Wilson, G. M. Tristetraprolin: roles in cancer and senescence. Ageing Res. Rev. 11, 473–484 (2012).
Ciais, D. et al. Destabilization of vascular endothelial growth factor mRNA by the zinc-finger protein TIS11b. Oncogene 23, 8673–8680 (2004).
Maitra, S. et al. The AU-rich element mRNA decay-promoting activity of BRF1 is regulated by mitogen-activated protein kinase-activated protein kinase 2. RNA 14, 950–959 (2008).
Ciais, D., Cherradi, N. & Feige, J. J. Multiple functions of tristetraprolin/TIS11 RNA-binding proteins in the regulation of mRNA biogenesis and degradation. Cell. Mol. Life Sci. 70, 2031–2044 (2013).
Benjamin, D., Schmidlin, M., Min, L., Gross, B. & Moroni, C. BRF1 protein turnover and mRNA decay activity are regulated by protein kinase B at the same phosphorylation sites. Mol. Cell. Biol. 26, 9497–9507 (2006).
Spasic, M. et al. Genome-wide assessment of AU-rich elements by the AREScore algorithm. PLoS Genet. 8, e1002433 (2012).
Tan, F. E. & Elowitz, M. B. Brf1 posttranscriptionally regulates pluripotency and differentiation responses downstream of Erk MAP kinase. Proc. Natl Acad. Sci. USA 111, E1740–E1748 (2014).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Franceschi, C. et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 (2007).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Barradas, M. et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev. 23, 1177–1182 (2009).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated β-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806 (2009).
Bishop, C. L. et al. Primary cilium-dependent and -independent Hedgehog signaling inhibits p16(INK4A). Mol. Cell 40, 533–547 (2010).
Larsen, M. R., Thingholm, T. E., Jensen, O. N., Roepstorff, P. & Jorgensen, T. J. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol. Cell. Proteomics 4, 873–886 (2005).
Montoya, A., Beltran, L., Casado, P., Rodriguez-Prados, J. C. & Cutillas, P. R. Characterization of a TiO2 enrichment method for label-free quantitative phosphoproteomics. Methods 54, 370–378 (2011).
Alcolea, M. P., Casado, P., Rodriguez-Prados, J. C., Vanhaesebroeck, B. & Cutillas, P. R. Phosphoproteomic analysis of leukemia cells under basal and drug-treated conditions identifies markers of kinase pathway activation and mechanisms of resistance. Mol. Cell. Proteomics 11, 453–466 (2012).
Hornbeck, P. V. et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 (2012).
Wilkinson, J. E. et al. Rapamycin slows aging in mice. Aging Cell 11, 675–682 (2012).
Miller, R. A. et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A 66, 191–201 (2011).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Acknowledgements
We are grateful to T. Rodríguez, A. Nebreda, R. Agami, M. Gaestel, D. Engelberg, G. Wendel, J. Campisi, C. Moroni, C. Speck, A. Day and G. Peters for reagents, helpful comments and contributions to this project. R. Miller is funded by NIH Grant U01-AG022303. Core support from MRC funded the research in J. Gil’s laboratory. N. Herranz was funded by an EMBO fellowship.
Author information
Authors and Affiliations
Contributions
N.H. and J.G. designed the experiments. N.H., S.G., M.M., C.J.H., S.R., J.C.A., A.J.I., A.B. and A.G. carried out and analysed the cell culture experiments. N.H., S.G., M.M., T.W., S.K., K.W., C.J.H. and F.R. carried out and analysed the animal experiments. N.H., S.G. and J.G. wrote the paper. P.F. and A.M. carried out the mass spectrometry experiments and analysis, supervised by P.C. G.D. and T.C. carried out the bioinformatics analysis. R.M. provided mouse samples and J.P.M.-B. provided reagents. All authors interpreted data, discussed results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
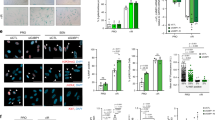
Supplementary Figure 1 mTOR inhibition regulates the SASP.
(a) mTOR inhibition regulates the SASP in different fibroblasts strains. BJ ER:RAS and HFFF2 ER:RAS cells were generated and induced with 4OHT to activate ER:RAS in the presence of 10 nM rapamycin, 25 nM Torin1 or 0.5 nM NVP-BEZ235. Seven days after 4OHT induction, RNA was collected and qPCRs performed to detect the expression of selected SASP components. Data are mean ± s.d. from n = 3 independent experiments. Student’s t-test was performed to compare the different treatments to the control (R + DMSO) with ***P < 0.001. V, cells expressing an empty vector; R, cells infected with pLNC-ER:RAS. (b,c) mTOR inhibition (b) or mTOR knockdown (c) decreases phosphorylation of S6 (pS6S240/S244) and eIF4EBP1 (p4EBP1T37/T46) proteins and inhibits the induction of the SASP. Indicated immunostainings were performed 7 days after 4OHT induction. Fold changes of normalised values and representative images are shown. Error bars represent the ±s.d. from n = 4 independent experiments. Student’s t-test was performed to compare treated/shMTOR cells to the control senescent cells. ***P < 0.001. Scale bar, 30 μm. (d) Venn diagrams representing the overlap and significance for the SASP components downregulated or upregulated by multiple mTOR inhibitors and mTOR knockdown. The significance of overlap was computed using hypergeometric test. **P < 0.01; *P < 0.05. For raw data, see Supplementary Table 7.
Supplementary Figure 2 mTOR inhibition controls the senescence phenotype and cell proliferation.
(a) mTOR inhibition decreases the levels of cell cycle inhibitors involved in senescence. p16INK4a and p21CIP1 levels were measured in the same experimental settings as in Supplementary Fig. 1b. Error bars represent the ±s.d. from n = 3 independent experiments. (b) mTOR knockdown results in decreased SA-β-Gal activity, p16INK4a and p21CIP1 levels but cells remain arrested. BrdU incorporation and senescence markers were determined after 7 days of induction with 4OHT. Data are mean ± s.d. from n = 6 (BrdU) and n = 4 (SA-β-Gal, p16 and p21) independent experiments. Student’s t-test was performed to compare the shmTOR cells with the senescent controls (shCT + 4OHT). ***P < 0.001; **P < 0.01: NS, non significant. (c) mTOR inhibition decreases proliferation of normal IMR90 human fibroblasts and does not rescue OIS arrest. IMR90 ER:RAS cells (2 × 104) were treated as indicated and proliferation was assessed by CV staining after 14 days in culture. Images are representative of 3 independent experiments. (d) Cyclin D3 expression levels were assessed by immunoblot. IMR90 ER:RAS cells were left untreated or treated with 4OHT and the indicated drugs for 2 or 8 days. Data are representative of n = 3 independent experiments. (e,f) These figures are an extension of the experiment presented in Fig. 2b, c. Torin1 inhibition of mTOR downstream targets (pS6S240/S244, p4EBP1T37/T46) and the SASP was assessed by immunoblot (e) and IF (f) 7 days after 4OHT induction. Immunoblots are representative of n = 3 independent experiments. Error bars represent the ±s.d. from n = 4 independent experiments. Student’s t-test was performed to compare Torin1 treated cells to the control. ***P < 0.001. Unprocessed original scans of blots are shown in Supplementary Fig. 9. For raw data, see Supplementary Table 7.
Supplementary Figure 3 Effect of mTOR and 4EBP1 on the translation of SASP components.
(a) This is an extension of Fig. 3a–c. Expression of an eIF4EBP1 phospho-mutant (4EBP1-DN) results in decreased phosphorylated 4EBP1Thr37/46 levels. Right: Fold changes of normalised intensity values obtained from 4EBP1 and 4EBP1Thr37/46 immunostainings Data are mean ± s.d. from n = 3 independent experiments. (left) Images from a representative experiment are shown. Data are mean ± s.d. from n = 3 independent experiments. For all graphs, Student’s t-test was performed to compare cells expressing a 4EBP1 DN to the control (+vector). ***P < 0.001. Scale bar, 30 μm. (b) Expression of 4EBP1-DN impairs the SASP levels observed in irradiated IMR90 fibroblasts. RNA was collected 8 days after irradiation (5 Gy). Data are mean ± s.d. from n = 3 independent experiments. Student’s t-test was performed to compare the cells expressing 4EBP1-DN to the control (vector irradiated). ***P < 0.001; *P < 0.05. (c) Profile of mTOR-regulated translation in OIS. Representative polysome profiles of IMR90 ER:RAS cells (incubated for 6 days with 4OHT) treated with DMSO or 250 nM Torin1 for 3 h from n = 3 independent experiments. (d) A schematic representation of the position of fractions (3–10), monosomes and polysomes is shown together with a representative profile for EEF2 mRNA (a canonical mTOR target). Data are representative of 3 independent experiments. (e) Polysome analysis of mRNAs for SASP components. Graphs show the percentage of the indicated mRNAs in actively translating polysomes during OIS. Error bars represent the ±s.d. from n = 3 independent experiments. Student’s t-test was performed to compare Torin1 treated cells to the DMSO-treated controls. ***P < 0.001; **P < 0.01; *P < 0.05; NS, non significant. For raw data, see Supplementary Table 7.
Supplementary Figure 4 MAPKAKP2 regulates the SASP.
(a) Polysome distribution of MKK6 mRNA. Error bars represent the ±s.d. from n = 3 independent experiments. Student’s t-test was performed to compare Torin1 treated cells to the control. NS, non significant. (b) Related to Fig. 4e. Samples were immunoblotted with Streptavidin-HRP to detect AHA-Biotin containing proteins. Samples purified by pull down with streptavidin beads (Strept. PD) were analysed. Data are representative of 3 independent experiments. (c) MAPKAPK2 protein levels correlate with mTOR signalling. IMR90 ER:RAS, BJ ER:RAS and HFFF2 ER:RAS cells were left untreated or treated with 4OHT for the indicated times. (Top) Immunoblots were performed with the indicated antibodies. Data are representative of 3 independent experiments. (Bottom) Quantification of MK2, pS6S240/S244 and p4EBP1T37/T46 blots. Error bars represent the ±s.d. from n = 3 independent experiments. (d) MAPKAPK2 inhibition prevents SASP induction. IMR90 ER:RAS cells were infected with the indicated hairpins and further treated with 4OHT. RNA and protein extracts were collected at day 6 after induction. Expression of the SASP and MK2 were assessed by qRT-PCR (left) or immunoblotting (right). Immunoblot images are representative of 3 independent experiments. Error bars represent the ±s.d. from n = 3 independent experiments. Student’s t-test was performed to compare cells deficient for MK2 to control cells (shCT + 4OHT). ***P < 0.001; **P < 0.01; *P < 0.05; NS, non significant. Unprocessed original scans of blots are shown in Supplementary Fig. 9. For raw data, see Supplementary Table 7.
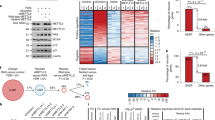
Supplementary Figure 5 ZFP36L1 regulates the SASP.
(a) Serine54 in ZFP36L1 was identified as an mTOR inhibition-sensitive phosphosite by phosphoproteomics (n = 3). (b) Extension of Fig. 5b. Specificity of MK2 inhibitor III is proved by the decrease in the phosphorylation of HSP27 (pHSP27S82) a downstream target of MK2. (c) Schematic model of ZFP36L1 activity and protein stability. (d) Percentage of ZFP36L1 mRNA in translating polysomes during OIS (n = 3). (e) IMR90 ER:RAS cells were infected as indicated. Immunostainings were performed 7 days after 4OHT induction. Fold changes of normalised intensity values are shown (n = 4). (f) SASP genes expression was measured in IMR90 fibroblasts 8 days after irradiation (5 Gy) (n = 3). (g) Designed TTP and ZFP36L2 phosphomutants. As MK2 phosphosites for TTP and ZFP36L2 have not been described, we aligned their sequences with that of ZFP36L1 aiming to identify putative MK2 phosphosites. In the case of TTP, homology is low. However, the two known MK2 phosphosites in the murine form of TTP (ref. 59) are conserved in the human form. (h,i) IMR90 ER:RAS cells were infected with ZFP36L1wt/mut, ZFP36L2wt/mut or TTPwt/mut. OIS was induced by 4OHT for 7 days. (h) Protein levels were analysed by immunoblot in presence of MG132 (2 μM, 12 h). (i) Expression of SASP genes was measured by qRT-PCR (n = 3). (j) SASP components detected in our proteomics analysis that are downregulated by mTORinhibitors, shmTOR and ZFP36L1Mut. (k) Venn diagrams representing the overlap between SASP factors upregulated by mTOR inhibitors, shmTOR and ZFP36L1Mut. (l) ARE Score for SASP factors: (left) commonly upregulated by shmTOR and mTOR inhibitors; (right) commonly downregulated by shmTOR, mTOR inhibitors and ZFP36L1Mut. Plots denote ARE score distribution for 105 random combinations of 15 and 17 mRNAs respectively. Statistical significance was calculated by using: (d,e,j and i): Student’s t-test (k: hypergeometric test), (l: permutation tests). ***P < 0.001; **P < 0.01; *P < 0.05; NS, non significant. Error bars represent means ± s.d. For b and h data are representative of 3 independent experiments. n represents number of independent experiments. For unprocessed original scans of blots, see Supplementary Fig. 9. For raw data, see Supplementary Table 7.
Supplementary Figure 6 Further characterization of the role of ZFP36L1 in the regulation of the SASP.
(a) GSEAs of a signature associated with senescence60 and (left) SASP components upregulated in OIS (ref. 6; right) in the gene expression profile (GEP) of IMR90 ER:RAS cells expressing ZFP36L1Mut versus control IMR90 ER:RAS, both 4OHT-treated. NES, normalized enrichment score; FDR, false discovery rate. GEPs were obtained by performing transcriptome sequencing (RNA-Seq) of the aforementioned conditions in n = 3 independent experiments. RNAs were collected 7 days after induction with 4OHT. (b) Mean ARE score of genes down regulated (at the indicated log2 of fold changes) when comparing IMR90 ER:RAS cells expressing ZFP36L1Mut versus control IMR90 ER:RAS, are shown in blue. The distribution of mean ARE scores derived from 105 simulations of random genes with equal sample size are shown in red. (c) This is an extension of Fig. 6c. The expression of TIMP was monitored by qRT-PCR at the indicated times. Data are for a representative experiment of n = 2 independent experiments. (d) Brdu incorporation was measured by IF at the indicated times. Error bars represent the ±s.d. from n = 3 independent experiments. Student’s t-test was performed to compare ZFP36L1Mut expressing cells (200 ng ml−1 doxycycline) to the control senescent cells (vector, +4OHT). ***P < 0.001. (e,f) IMR90 ER:RAS expressing TRE–FLAG-ZFP36L1Mut (or empty vector) and the reverse transactivator (rtTA-M3) were further infected with a vector encoding the coding sequence of p21. After 6 days of incubation with 4OHT, the expression of ZFP36L1Mut was induced with 200 ng ml−1 doxycycline for 72 h and (e) the % of BrdU positive cells or (f) SA-β-Gal positive cells was quantified. Error bars represent the ±s.d. from n = 4 independent experiments. SA-β-Gal stainings are representative of n = 3 independent experiments. Student’s t-test was performed to compare the indicated conditions ***P < 0.001; NS, non significant. For raw data, see Supplementary Table 7.
Supplementary Figure 7 The paracrine pro-tumorigenic effects of senescent cells are sensitive to mTOR inhibition and ZFP36L1 activity.
(a) mTOR inhibition prevents the EMT induced by the CM of senescent cells. IMR90 ER:RAS cells were treated as indicated in the scheme. CM was collected 7 days after 4OHT induction (mTOR inhibitors were removed from the media at day 4, see Methods for details) and T47D cells cultured in that CM. 48 h after, E-Cadherin expression was monitored by IF. Fold changes of normalised intensity values (top) and representative pictures are shown (bottom). Data are mean ± s.d. from n = 4 independent experiments. Student’s t-test was performed to compare treated cells to the control. ***P < 0.001. Scale bar, 40 μm. (b) Both inhibition of mTOR signalling and expression of ZFP36L1Mut prevent the upregulation of EMT-specific transcription factors by the CM of senescent cells. IMR90 ER:RAS cells were treated as indicated in the scheme in (a). CM was collected after 7 days. T47D cells were cultured in that CM for the indicated times and processed for RNA extraction. Snail1 and Zeb1 mRNA expression levels were measured by qRT-PCR. In the case of the CM of IMR90 ER:RAS treated with mTOR inhibitors, data are mean ± s.d. from n = 3 independent experiments. For the rest of the experiments data are from a representative experiment of n = 2 independent experiments. (c) CM of both proliferating and arrested ZFP36L1Mut expressing cells is unable to trigger a complete EMT. The indicated cells were treated with 4OHT for 9 days. Expression of TRE–ZFP36L1Mut was activated for the last 72 h. CM was then collected and T47D cells were cultured in that CM for 48 h. E-Cadherin expression was monitored by IF. Fold changes of normalised intensity values (right) and representative pictures are shown (left). Data are mean ± s.d. from n = 3 independent experiments. Student’s t-test was performed to compare ZFP36L1Mut cells to the control (Vector/ Vector). **P < 0.01. Scale bar, 40 μm. For raw data, see Supplementary Table 7.
Supplementary Figure 8 mTOR inhibition blunts the tumour suppressive effects of the SASP.
(a) CMs of IMR90 ER:RAS expressing TRE– ZFP36L1Mut (or empty vector) and p21 (or empty vector) were collected 72 h after induction of ZFP36L1Mut and 9 days after 4OHT addition. The effect CMs had on BrdU incorporation and SASP induction in IMR90 wt cells was evaluated by IF. Graphs show data from n = 4 independent experiments. (b) IMR90 wt cells were co-cultured with the indicated IMR90 ER:RAS cells with or without 4OHT. BrdU incorporation was monitored by IF. Data are representative of n = 2 independent experiments. (c) This figure is related to Fig. 8c. Expression of the indicated transcripts was quantified by qRT-PCR. Carrier, n = 5 mice; Rapamycin, n = 4 mice. P-values are included. (d) Expression of MAPKAPK2, 4EBP1 and phosphorylation of 4EBP1 were analysed by immunoblot. Quantification is presented in Fig. 8d. (e) NrasG12V transposons were co-delivered into mouse livers through hydrodynamic injection (Day 0). Mice were sacrificed 3 days later and Nras+ cells were counted (n = 4 mice per condition) Mice were treated with carrier or drugs from 3 days prior NrasG12V injection. Representative images of the sections quantified are shown (200x). Scale bar, 100 μm. (f) Quantification of the expression of immune cell markers by qRT-PCR in livers of the indicated mice 6 days after NrasG12V injection. Carrier, n = 5 mice; rapamycin, n = 4 mice. CD3, T cell marker; B220, B cell marker; CD68, macrophage marker; KlrD, NK cell marker. P values are included. (g) NrasG12V transposons were co-delivered into mice as explained before. Mice were sacrificed 12 days later. Mice were only treated with carrier or rapamycin 3 days post-injection Nras+ (left), p21Cip1+ (centre) and p16Ink4a+ (right) cells on liver sections were counted (n = 4 mice per condition). Representative images of sections quantified in the top panel are shown in the bottom. Scale bar, 100 μm. Statistical significance was calculated using Student’s t-test, ***P < 0.001; **P < 0.01; *P < 0.05; NS, non significant. Error bars represent means ± s.d. For unprocessed original scans of blots, see Supplementary Fig. 9. For raw data, see Supplementary Table 7.
Supplementary information
Supplementary Information
Supplementary Information (PDF 7809 kb)
Supplementary Table 1
Supplementary Information (XLS 38 kb)
Supplementary Table 2
Supplementary Information (XLSX 88 kb)
Supplementary Table 3
Supplementary Information (XLSX 2425 kb)
Supplementary Table 4
Supplementary Information (XLSX 34 kb)
Supplementary Table 5
Supplementary Information (XLSX 39 kb)
Supplementary Table 6
Supplementary Information (XLSX 39 kb)
Supplementary Table 7
Supplementary Information (XLSX 225 kb)
Rights and permissions
About this article
Cite this article
Herranz, N., Gallage, S., Mellone, M. et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17, 1205–1217 (2015). https://doi.org/10.1038/ncb3225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3225
This article is cited by
-
MicroRNA-377-3p exacerbates chronic obstructive pulmonary disease through suppressing ZFP36L1 expression and inducing lung fibroblast senescence
Respiratory Research (2024)
-
Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature
European Journal of Medical Research (2024)
-
Low expression of ZFP36L1 in osteosarcoma promotes lung metastasis by inhibiting the SDC4-TGF-β signaling feedback loop
Oncogene (2024)
-
Aging microenvironment and antitumor immunity for geriatric oncology: the landscape and future implications
Journal of Hematology & Oncology (2023)
-
mTOR inhibition abrogates human mammary stem cells and early breast cancer progression markers
Breast Cancer Research (2023)