Abstract
Pathological expansion of adipose tissue contributes to the metabolic syndrome. Distinct depots develop at various times under different physiological conditions. The transcriptional cascade mediating adipogenesis is established in vitro, and centres around a core program involving PPARγ and C/EBPα. We developed an inducible, adipocyte-specific knockout system to probe the requirement of key adipogenic transcription factors at various stages of adipogenesis in vivo. C/EBPα is essential for all white adipogenic conditions in the adult stage, such as adipose tissue regeneration, adipogenesis in muscle and unhealthy expansion of white adipose tissue during high-fat feeding or due to leptin deficiency. Surprisingly, terminal embryonic adipogenesis is fully C/EBPα independent, but does however depend on PPARγ; cold-induced beige adipogenesis is also C/EBPα independent. Moreover, C/EBPα is not vital for adipocyte survival in the adult stage. We reveal a surprising diversity of transcriptional signals required at different stages of adipogenesis in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Rosen, E. D. & Spiegelman, B. M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 (2006).
Cinti, S. The adipose organ at a glance. Dis. Models Mech. 5, 588–594 (2012).
Abate, N., Garg, A., Peshock, R., Stray-Gunderson, J. & Grundy, S. Relationship of generalized and original adiposity to insulin sensitivity in men. J. Clin. Invest. 96, 88–98 (1995).
Miyazaki, Y. & DeFronzo, R. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc. Diabetol. 8, 44 (2009).
McLaughlin, T., Lamendola, C., Liu, A. & Abbasi, F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 96, E1756–E1760 (2011).
Gesta, S., Tseng, Y.-H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007).
Cristancho, A. G. & Lazar, M. A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734 (2011).
Spiegelman, B. M. & Flier, J. S. Obesity and the regulation of energy balance. Cell 104, 531–543 (2001).
Mikkelsen, T. S. et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143, 156–169 (2010).
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 (2006).
Rosen, E. D. & MacDougald, O. A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 (2006).
Lefterova, M. I. et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 22, 2941–2952 (2008).
Nielsen, R. et al. Genome-wide profiling of PPARγ:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 22, 2953–2967 (2008).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Gupta, R. K. et al. Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623 (2010).
Jeffery, E., Church, C. D., Holtrup, B., Colman, L. & Rodeheffer, M. S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 17, 376–385 (2015).
Berry, R., Jeffery, E. & Rodeheffer, M. S. Weighing in on adipocyte precursors. Cell Metab. 19, 8–20 (2014).
Wang, Z. V., Deng, Y., Wang, Q. A., Sun, K. & Scherer, P. E. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151, 2933–2939 (2010).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. & Whitsett, J. A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl Acad. Sci. USA 99, 10482–10487 (2002).
Zhang, P. et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPα. Immunity 21, 853–863 (2004).
Wu, Z., Xie, Y., Bucher, N. & Farmer, S. R. Conditional ectopic expression of C/EBP β in NIH-3T3 cells induces PPAR γ and stimulates adipogenesis. Genes Dev. 9, 2350–2363 (1995).
Hamm, J. K., Park, B. H. & Farmer, S. R. A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 276, 18464–18471 (2001).
Rosen, E. D. et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16, 22–26 (2002).
Wu, Z. et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3, 151–158 (1999).
Linhart, H. G. et al. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc. Natl Acad. Sci. USA 98, 12532–12537 (2001).
Wang, N. D. et al. Impaired energy homeostasis in C/EBPα knockout mice. Science 269, 1108–1112 (1995).
Chatterjee, R. et al. Suppression of the C/EBP family of transcription factors in adipose tissue causes lipodystrophy. J. Mol. Endocrinol. 46, 175–192 (2011).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
He, W. et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl Acad. Sci. USA 100, 15712–15717 (2003).
Wang, F., Mullican, S. E., DiSpirito, J. R., Peed, L. C. & Lazar, M. A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl Acad. Sci. USA 110, 18656–18661 (2013).
Imai, T. et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl Acad. Sci. USA 101, 4543–4547 (2004).
Yang, J. et al. Metabolic response of mice to a postnatal ablation of CCAAT/Enhancer-binding protein α. J. Biol. Chem. 280, 38689–38699 (2005).
Holland, W. L. & Summers, S. A. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29, 381–402 (2008).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2010).
Wang, Q. A., Scherer, P. E. & Gupta, R. K. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J. Lipid Res. 55, 605–624 (2014).
Kim, J.-Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Pajvani, U. B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11, 797–803 (2005).
Joe, A. W. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 (2010).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Chau, Y. Y. et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 16, 367–375 (2014).
Sanchez-Gurmaches, J. & Guertin, D. A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099 (2014).
Macotela, Y. et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61, 1691–1699 (2012).
Tchkonia, T. et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 (2013).
Baglioni, S. et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 7, e36569 (2012).
Park, S. K. et al. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes 53, 2757–2766 (2004).
Qiao, L. et al. C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54, 1744–1754 (2005).
Qiao, L., Schaack, J. & Shao, J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology 147, 865–874 (2006).
Maeda, N. et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 (2002).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Ye, R. et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife 3, e03851 (2014).
Ye, R. & Scherer, P. E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2, 133–141 (2013).
Asterholm, I. W. & Scherer, P. E. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J. Pathol. 176, 1364–1376 (2010).
Shetty, S., Kusminski, C. M. & Scherer, P. E. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol. Sci. 30, 234–239 (2009).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Wang, Q. et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 49, 1166–1175 (2009).
Wang, Q. et al. Deficiency in hepatic ATP-citrate lyase affects VLDL-triglyceride mobilization and liver fatty acid composition in mice. J. Lipid Res. 51, 2516–2526 (2010).
Lukjanenko, L., Brachat, S., Pierrel, E., Lach-Trifilieff, E. & Feige, J. N. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS ONE 8, e71084 (2013).
Acknowledgements
We thank the UT Southwestern Histology Core for assistance in embedding and processing of tissue samples. We would also like to thank Shimadzu Scientific Instruments for the collaborative effort and advice on the mass spectrometric instrumentation side. This study was supported by US National Institutes of Health (NIH) grants R01-DK55758, P01-DK088761 and R01-DK099110 (P.E.S.). Q.A.W. is supported by a postdoctoral fellowship from the American Diabetes Association (7-11-MN-47). R.K.G. is supported by NIH grants R01-DK104789 and R03-DK099428 and the Searle Scholars Program (Chicago, IL).
Author information
Authors and Affiliations
Contributions
Q.A.W., R.K.G. and P.E.S. designed the experiments and wrote the manuscript. Q.A.W., C.T., L.J., M.S., R.Y., Y.Z., R.G., A.A. and R.K.G. performed experiments and analysed data. Y. L. and W.L.H. analysed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
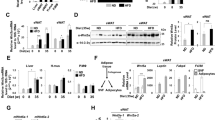
Supplementary Figure 1 Adipocyte specific inducible knockout of C/EBPα in maturing and mature adipocytes.
a. Inducible, mature adipocytes specific knockout of C/EBPα. In the Adn-C/EBPαflox/flox mice, C/EBPα is expressed normally in every cell. On doxycycline treatment, rtTA will activate the TRE promoter to induce Cre expression. Cre protein will subsequently cut the floxed region in C/EBPα and eliminate the expression of C/EBPα in all existing mature adipocytes (Adn-C/EBPα−/−). Mice lacking TRE–Cre or Adn-rtTA were used as controls. b. Tissue profile of C/EBPα mRNA expression by qPCR analysis after 7 days of doxycycline chow diet feeding. n = 4 male mice (control group), n = 2 male mice (Adn-C/EBPα−/− group). This experiment is representative of two independent experiments. c. qPCR analysis of C/EBPα mRNA levels in the adipocyte fraction and SVF fraction of sWAT and eWAT (left) and of C/EBPβ mRNA level in the adipocyte fraction of sWAT and eWAT (right) after 7 days of doxycycline chow diet feeding. n = 3 male mice per group. **, P < 0.001 for sWAT adipocyte; *, P = 0.04 for eWAT adipocyte. This experiment is representative of two independent experiments. d. Western-blots of C/EBPα andβ-actin levels in protein extracts of sWAT, eWAT and liver from Adn-C/EBPα−/− mice and their control littermates after 4 days of doxycycline chow diet feeding. These images are representative of two independent western-blots experiments. e. qPCR analysis of C/EBPα, PPARγ, adiponectin and rtTA mRNA levels during day 0–day 6 of SVF differentiation. SVF is extracted from sWAT of Adn-C/EBPαflox/flox mice. These images are from a single experiments. f. Oil Red O staining of SVF differentiation (extracted from sWAT of Adn-C/EBPαflox/flox mice) on days 0–6, as indicated. These images are representative of two independent western-blots experiments. g. CHIP assay in adipocytes differentiated from SVF of Adn-C/EBPαflox/flox mice. Starting from the 8th way of differentiation, doxycycline (10 μg ml−1) was added to the cell growth medium for 4 days to generate C/EBPα free adipocytes and medium was switched to doxycycline free for another two days of before sample collection. CHIP was performed with C/EBPα (left), C/EBPβ (middle) and PPARγ (right) antibodies and the enrichments of Cd36 and C/ebpβ promoter in the immunoprecipitated DNA were tested by qPCR analysis, using 18s as a quality control. n = 310 cm dishes per group for C/EBPα CHIP; n = 310 cm dishes per group for PPARγ CHIP, Cd36; n = 210 cm dishes (control group), n = 310 cm dishes (Adn-C/EBPα−/− group) for PPARγ CHIP, C/ebpβ. n = 210 cm dishes per group for C/EBPβ CHIP. *, P = 0.02 compared to control group. All data represent the mean or mean ± s.e.m. Student’s t-test. This data is from a single experiment.
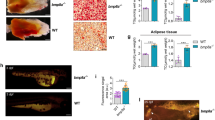
Supplementary Figure 2 C/EBPα expression is not required for late embryonic adipogenesis of sWAT and BAT.
a,b. Immunofluorescence stains for C/EBPα (green), DAPI (blue) and perilipin (red) in adipocyte nucleus in eWAT (a) or sWAT (b) of 7 weeks old Adn-C/EBPα−/−(E11−P16) male mice and their control male littermates. These images are representative of two independent experiments. c. Whole tissue pictures of pWAT and sWAT from Adn-C/EBPα−/−(E11−P16) female mice and their control female littermates at 7 weeks of age. These images are from a single experiment. d. H&E staining of sWAT and pWAT in Adn-C/EBPα−/−(E11−P16) female mice and their control female littermates. These images are from a single experiment. e. Perilipin (red) and DAPI (blue) immunofluorescence staining of sWAT and pWAT in Adn-C/EBPα−/−(E11−P16) female mice and their control female littermates. These images are from a single experiment. f. Western-blots of C/EBPα andβ-actin levels in protein extracts of BAT from Adn-C/EBPα−/−(E11−P16) male or female mice and their control littermates at 7 weeks of age. These images are from a single Western-blot experiment. g. Representative H&E stains of BAT in Adn-C/EBPα−/−(E11−P16) male (left) or female mice (right) and their control littermates at 7 weeks of age. These images are from a single experiment. h. Representative perilipin (red) and DAPI (blue) immunofluorescence stains of BAT in Adn-C/EBPα−/−(E11−P16) male (left) or female mice (right) and their control littermates at 7 weeks of age. These images are from a single experiment.
Supplementary Figure 3 C/EBPα is not required for terminal development of eWAT.
a. Experimental design: Adn-C/EBPαflox/flox or control littermates (mice contains only Adn-rtTA and C/EBPαflox/flox) were on doxycycline chow diet during P0–P42. b. sWAT and eWAT tissue mass tissue mass of Adn-C/EBPα−/−(P0−P42) male mice and their control male littermates at 8 weeks of age. n = 4 male mice (control group), n = 3 male mice (Adn-C/EBPα−/−(P0−P42) group). Data represent the mean ± s.e.m. This data is from a single experiment. c. Whole tissue pictures of eWAT and sWAT from Adn-C/EBPα−/−(P0−P42) male mice and their control male littermates. These images are from a single experiment. d. Western-blot of C/EBPα levels in protein extracts of eWAT and sWAT from Adn-C/EBPα−/−(P0−P42) male mice and their control male. These images are from a single Western-blot experiment. e. Experimental design: Adn-C/EBPαflox/flox or control littermates (mice contains only Adn-rtTA and C/EBPαflox/flox) were on doxycycline chow diet during E11–P42. f. sWAT and eWAT tissue mass of Adn-C/EBPα−/−(E11−P42) male mice and their control male littermates at 8 weeks of age. n = 4 male mice per group. Data represent the mean ± s.e.m. This data is from a single experiment. g. Whole tissue pictures of eWAT and sWAT from Adn-C/EBPα−/−(E11−P42) male mice and their control male littermates. These images are from a single experiment.
Supplementary Figure 4 C/EBPα and PPARγ specific transcriptional programs in mature adipocytes.
a. Immediate PPARγ-responsive genes clustered and presented as a heat map, based on the log base 2-transformed expression levels for these genes. Adn-PPARγ−/− mice and their control littermates were on 3 days of doxycycline chow feeding (Fig. 3g). P cut-off: 0.05; fold change cut-off: 2. This data is from a single experiment. b. C/EBPα direct responsive genes clustered and presented as a heat map. Adn-C/EBPα−/− mice and their control littermates were on 3 days of doxycycline chow feeding. P cut-off: 0.05; fold change cut-off: 1.5. This data is from a single experiment. c. Experimental design: 10 weeks old Adn-C/EBPαflox/flox male mice and their control male littermates were kept on doxycycline HFD for 3 days or 1 month to generate Adn-C/EBPα−/− mice. RNA from sWAT of all groups after 3 days of doxycycline chow feeding was extracted for microarray analysis. d,e. C/EBPα direct responsive genes clustered and presented by heat map. Adn-C/EBPα−/− mice and their control littermates were on 3 days of doxycycline HFD feeding (d) or 1 month of doxycycline HFD feeding (e) P cut-off: 0.05; fold change cut-off: 1.5. For each group, RNA from each mouse was pooled into 3 samples for microarray analysis. n = 10 male mice per group, HFD 3 days; n = 9 male mice per group, HFD 1 month. This data is from a single experiment.
Supplementary Figure 5 Mature adipocyte specific inducible deletion of C/EBPα alters whole body lipid metabolism during HFD feeding.
a,b. Lipoprotein profiles determined using pooled serum samples by FPLC fractionation from Adn-C/EBPα−/− mice and their control littermates at the 4th week of doxycycline HFD feeding. Triglyceride (a) and cholesterol (b) concentrations were measured in each indicated fraction corresponding to VLDL, IDL/LDL and HDL, respectively (left). The relative triglyceride content in VLDL fractions (a) and cholesterol content in HDL fractions (b) are also shown using the areas under curve from the FPLC profiles (right). n = 6 male mice per group. This experiment is representative of two independent experiments. c. Triglyceride clearance test (20% intralipid, 10 μl per gram of body weight, single gavage) in Adn-C/EBPα−/− mice and their control littermates. n = 9 male mice (HFD control group), n = 5 male mice (HFD Adn-C/EBPα−/− group). **, P < 0.001, compared to HFD control group. Two-way ANOVA. This data is from a single experiment. d. Triglyceride secretion rate were measured in Adn-C/EBPα−/− mice and their control littermates after tyloxapol administration (through tail-vein injection, 600 mg kg−1 body weight). Blood triglycerides were measured at the time points as indicated (left), and the VLDL secretion rate (right) was derived from the slope of the line using least squares regression. n = 3 male mice (HFD control group), n = 4 male mice (HFD Adn-C/EBPα−/− group). **, P = 0.002, < 0.001 for triglyceride levels at 3 h and 4 h; Two-way ANOVA. *, P = 0.008 for VLDL secretion rate, compared to HFD control group. Student’s t-test. This data is from a single experiment. e–g. Circulating NEFA (e), triglyceride (f) and glucose (g) in Adn-C/EBPα−/− mice and their control littermates at 5th week of doxycycline HFD feeding after β3 agonist treatment (1 mg/kg, body weight). n = 7 male mice (HFD control group), n = 12 male mice (HFD Adn-C/EBPα−/− group). *, P = 0.02 for triglyceride level at 120 min; *, P = 0.04, 0.02 for glucose levels at 30 and 60 min, compared to HFD control group. Two-way ANOVA. This data is from a single experiment. h,i. Hepatic triglyceride (TG) (h) and cholesterol (CHO) (i) levels in Adn-C/EBPα−/− mice and their control littermates after 4 weeks of doxycycline HFD feeding. n = 12 male mice (HFD control group), n = 11 male mice (HFD Adn-C/EBPα−/− group). This data is from a single experiment. All data represent the mean ± s.e.m. j–m. Physical activity (j), oxygen consumption (k), RER (l) and food intake (m) were determined for Adn-C/EBPα−/− mice and their control littermates during the 7th week of doxycycline HFD feeding. n = 6 male mice per group. XA: X beam activities; YA: Y beam activities; CenA: Central beam activities. This data is from a single experiment.
Supplementary Figure 6 Inducible deletion of C/EBPα in mature adipocytes during HFD feeding alters ceramide content and expressions of key transcription factors, adipokines and enzymes in WAT.
a–i. Sphingoid base contents were measured by LC/MS/MS method in liver (a), sWAT (d) and eWAT (g) of Adn-C/EBPα−/− mice and their control littermates after 1 month of doxycycline HFD feeding. Ceramides contents were measured by LC/MS/MS method in liver (b), sWAT (e) and eWAT (h) of Adn-C/EBPα−/− mice and their control littermates after 1 month of doxycycline HFD feeding. Sphingomyelin and Ceramide content was measured by LC/MS/MS method in liver (c), sWAT (f) and eWAT (i) of Adn-C/EBPα−/− mice and their control littermates after 1 month of doxycycline HFD feeding. n = 11 male mice (HFD control group), n = 12 male mice (HFD Adn-C/EBPα−/− group). Data represent the mean ± s.e.m. (a) **, P = 0.004; (b) from left to right: *, P = 0.01, **, P = 0.007, *, P = 0.04; (d) from left to right: *, P = 0.04, **, P < 0.001; (e) from left to right: **, P < 0.001, **, P < 0.001, **, P < 0.001, **, P < 0.001, **, P < 0.001, *, P = 0.03, **, P = 0.006, **, P < 0.001, *, P = 0.01; (f) from left to right: *, P = 0.02, **, P < 0.001; (g) from left to right: **, P < 0.001, *, P = 0.02, **, P < 0.001; (h) from left to right: *, P = 0.04, *, P = 0.02, **, P < 0.001, **, P < 0.001, **, P = 0.002, *, P = 0.03, *, P = 0.02; (i) *, P = 0.01. All compared to HFD control group. Student’s t-test. This data is from a single experiment. j–l. mRNA levels of key transcription factors were measured by qPCR, including PPARγ, SREBP1c, SREBP2, ChREBP, LXRα, LXRβ and PGC1-α (j); adipocyte markers adiponectin (ADPN), resistin, CD36, FATP1, CPT1α and Acox1 (k); lipogenic enzymes FAS, ACC, SCD-1 and Elovl6 (l) in sWAT of Adn-C/EBPα−/− mice and their control littermates after 4 weeks of doxycycline HFD feeding. n = 13 male mice per group. Data represent the mean ± s.e.m. *, P = 0.04 for Elovl6; **, P < 0.001 for PPARγ, LXRα, adiponectin, resistin, CD36 and SCD-1. All compared to HFD control group. Student’s t-test. This data is from a single experiment.
Supplementary Figure 7 Long-term, but not acute C/EBPα deletion on HFD shares common pathways with acute PPARγ deletion on chow diet.
Comparison analysis across the Canonical Pathway Analysis based on the four groups of arrays is shown by heat maps based on z-score. As indicated in Supplementary Fig. 4, the four groups are Adn-PPARγ−/−. mice after 3 days of doxycycline chow treatment; Adn-C/EBPα−/− mice after 3 days of doxycycline chow treatment, 3 days of doxycycline HFD or 1 month of doxycycline HFD. This data is from a single experiment.
Supplementary Figure 8 Mature adipocyte specific inducible C/EBPα knockout alters whole body glucose and lipid metabolism in ob/ob mice.
a. Magnetic resonance imaging analysis from representative ob/ob Adn-C/EBPα−/− mice and their ob/ob control littermates at the 8th week of doxycycline chow diet feeding. These images are from a single Western-blot experiment. b. GTT were performed during the 8th week of doxycycline chow diet feeding on ob/ob Adn-C/EBPα−/− mice and their control littermates, the bar graph represents the area under the curve for the blood glucose levels from glucose tolerance test measurements. n = 5 male mice per group. *, P = 0.02 for GTT 60 min, two-way ANOVA. *, P = 0.03 for GTT AUC compared to ob/ob control group, Student’s t-test. All data represent the mean ± s.e.m. This data is from a single experiment. c. Triglyceride secretion rates were measured on ob/ob Adn-C/EBPα−/− mice and their ob/ob control littermates after tyloxapol administration (through tail-vein injection, 600 mg kg−1 body weight). Blood triglycerides were measured at the time points as indicated (left), and the VLDL secretion rate (right) was derived from the slope of the line using least squares regression. n = 4 male mice (ob/ob control grouP), n = 5 male mice (ob/ob Adn- C/EBPα−/− grouP). **, P = 0.003, < 0.001 for triglycerides levels at 3 h and 4 h, two-way ANOVA; *, P = 0.03 for VLDL secretion rate, compared to ob/ob control group, Student’s t-test. All data represent the mean ± s.e.m. This data is from a single experiment. d–f. Oxygen consumption (d), RER (e) and physical activity (f) were determined for ob/ob Adn-C/EBPα−/− mice and their ob/ob control littermates during the 6th week of doxycycline HFD feeding. n = 6 male mice per group. Data represent the mean ± s.e.m. This data is from a single experiment.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2502 kb)
Supplementary Table 1
Supplementary Information (XLSX 25 kb)
Supplementary Table 2
Supplementary Information (XLSX 12 kb)
Supplementary Table 3
Supplementary Information (XLSX 17 kb)
Supplementary Table 4
Supplementary Information (XLSX 29 kb)
Supplementary Table 5
Supplementary Information (PDF 68 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Tao, C., Jiang, L. et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17, 1099–1111 (2015). https://doi.org/10.1038/ncb3217
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3217
This article is cited by
-
Maternal obesity and programming of metabolic syndrome in the offspring: searching for mechanisms in the adipocyte progenitor pool
BMC Medicine (2023)
-
Characteristic and fate determination of adipose precursors during adipose tissue remodeling
Cell Regeneration (2023)
-
Lipogenic Potency of Individual Perfluorinated Alkyl Acids (PFAAs) and Persistent Organic Pollutant (POP) Mixtures at Human Blood-Based Exposure Levels on Adipogenesis in 3T3-L1 Cells
Exposure and Health (2022)
-
Regulatory roles and mechanisms of alternative RNA splicing in adipogenesis and human metabolic health
Cell & Bioscience (2021)
-
Transcriptional networks controlling stromal cell differentiation
Nature Reviews Molecular Cell Biology (2021)