Abstract
We describe robust induction of autophagy during the reprogramming of mouse fibroblasts to induced pluripotent stem cells by four reprogramming factors (Sox2, Oct4, Klf4 and c-Myc), henceforth 4F. This process occurs independently of p53 activation, and is mediated by the synergistic downregulation of mechanistic target of rapamycin complex 1 (mTORC1) and the induction of autophagy-related genes. The 4F coordinately repress mTORC1, but bifurcate in their regulation of autophagy-related genes, with Klf4 and c-Myc inducing them but Sox2 and Oct4 inhibiting them. On one hand, inhibition of mTORC1 facilitates reprogramming by promoting cell reshaping (mitochondrial remodelling and cell size reduction). On the other hand, mTORC1 paradoxically impairs reprogramming by triggering autophagy. Autophagy does not participate in cell reshaping in reprogramming but instead degrades p62, whose accumulation in autophagy-deficient cells facilitates reprogramming. Our results thus reveal a complex signalling network involving mTORC1 inhibition and autophagy induction in the early phase of reprogramming, whose delicate balance ultimately determines reprogramming efficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Cai, J. et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. 2, 1–8 (2013).
Gonzalez, F., Boue, S. & Izpisua Belmonte, J. C. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat. Rev. Genet. 12, 231–242 (2011).
Vierbuchen, T. & Wernig, M. Molecular roadblocks for cellular reprogramming. Mol. Cell 47, 827–838 (2012).
Buganim, Y., Faddah, D. A. & Jaenisch, R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 14, 427–439 (2013).
Samavarchi-Tehrani, P. et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 (2010).
Rais, Y. et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature 502, 65–70 (2013).
Di Stefano, B. et al. C/EBPα poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature 506, 235–239 (2014).
Murrow, L. & Debnath, J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu. Rev. Pathol. 8, 105–137 (2013).
Choi, A. M., Ryter, S. W. & Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 1845–1846 (2013).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 22, 519–523 (2008).
Tanida, I. Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 14, 2201–2214 (2011).
Warlich, E. et al. Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol. Ther. 19, 782–789 (2011).
Carey, B. W., Markoulaki, S., Beard, C., Hanna, J. & Jaenisch, R. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat. Methods 7, 56–59 (2010).
Tsuneoka, M. et al. c-myc induces autophagy in rat 3Y1 fibroblast cells. Cell Struct. Funct. 28, 195–204 (2003).
Cho, Y. Y. et al. Autophagy and cellular senescence mediated by Sox2 suppress malignancy of cancer cells. PLoS ONE 8, e57172 (2013).
Nazio, F. et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406–416 (2013).
Gao, W., Shen, Z., Shang, L. & Wang, X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ. 18, 1598–1607 (2011).
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009).
Chen, J. et al. BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell Res. 21, 205–212 (2011).
Esteban, M. A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Shoji-Kawata, S. et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206 (2013).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Folmes, C. D. et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14, 264–271 (2011).
Wang, S. et al. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell 13, 617–625 (2013).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Cunningham, J. T. et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450, 736–740 (2007).
Morita, M. et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 18, 698–711 (2013).
He, J. et al. An elaborate regulation of Mammalian target of rapamycin activity is required for somatic cell reprogramming induced by defined transcription factors. Stem Cells Dev. 21, 2630–2641 (2012).
Tahmasebi, S. et al. Multifaceted regulation of somatic cell reprogramming by mRNA translational control. Cell Stem Cell 14, 606–616 (2014).
Inoki, K., Li, Y., Xu, T. & Guan, K. L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 (2003).
Tee, A. R., Manning, B. D., Roux, P. P., Cantley, L. C. & Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 (2003).
Budanov, A. V. & Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460 (2008).
Zhang, H. et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J. Clin. Invest. 112, 1223–1233 (2003).
Moscat, J. & Diaz-Meco, M. T. p62: a versatile multitasker takes on cancer. Trends Biochem. Sci. 37, 230–236 (2012).
Johansen, T. & Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 (2011).
Pattingre, S. et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, 927–939 (2005).
Wang, T. et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 9, 575–587 (2011).
Ji, J. et al. Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Rep. 2, 44–51 (2014).
Soufi, A., Donahue, G. & Zaret, K. S. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell 151, 994–1004 (2012).
Liu, X. et al. Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT–MET mechanism for optimal reprogramming. Nat. Cell Biol. 15, 829–838 (2013).
Vazquez-Martin, A. et al. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 11, 974–989 (2012).
Xu, X. et al. Stress-mediated p38 activation promotes somatic cell reprogramming. Cell Res. 23, 131–141 (2013).
March, H. N. & Winton, D. J. mTOR regulation by JNK: rescuing the starving intestinal cancer cell? Gastroenterology 140, 1387–1391 (2011).
Tang, H. W. et al. Atg9 interacts with dTRAF2/TRAF6 to regulate oxidative stress-induced JNK activation and autophagy induction. Dev. Cell 27, 489–503 (2013).
Liu, L. et al. Transcriptional pause release is a rate-limiting step for somatic cell reprogramming. Cell Stem Cell 15, 574–588 (2014).
Chen, J. et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat. Genet. 45, 34–42 (2013).
Kuo, T. C. et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat. Cell Biol. 13, 1214–1223 (2011).
Cho, Y. H. et al. Autophagy regulates homeostasis of pluripotency-associated proteins in hESCs. Stem Cells 32, 424–435 (2014).
Buganim, Y. et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell 13, 295–309 (2014).
Planello, A. et al. Aberrant DNA methylation reprogramming during induced pluripotent stem cell generation is dependent on the choice of reprogramming factors. Cell Regen. 3, 4–17 (2014).
Urabe, M., Kume, A., Tobita, K. & Ozawa, K. DNA/Calcium phosphate precipitates mixed with medium are stable and maintain high transfection efficiency. Anal. Biochem. 278, 91–92 (2000).
Wu, Y., Li, Y., Qin, B. & Pei, D. Reprogramming mouse embryonic fibroblasts using different systems. Protoc. Exch. http://dx.doi.org/10.1038/protex.2015.001 (2015)
Pyo, J. O. et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 280, 20722–20729 (2005).
Row, P. E., Reaves, B. J., Domin, J., Luzio, J. P. & Davidson, H. W. Overexpression of a rat kinase-deficient phosphoinositide 3-kinase, Vps34p, inhibits cathepsin D maturation. Biochem. J. 353, 655–661 (2001).
Tomoda, T., Bhatt, R. S., Kuroyanagi, H., Shirasawa, T. & Hatten, M. E. A mouse serine/threonine kinase homologous to C. elegans UNC51 functions in parallel fiber formation of cerebellar granule neurons. Neuron 24, 833–846 (1999).
Fujita, N. et al. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 19, 2092–2100 (2008).
Ho, J. C. et al. Generation of induced pluripotent stem cell lines from 3 distinct laminopathies bearing heterogeneous mutations in lamin A/C. Aging 3, 380–390 (2011).
Zhang, H. et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903 (2008).
Acknowledgements
We thank W. Lee and J. CY. Ho from the University of Hong Kong for their technical support with the electron microscopy. We thank all members of the Pei and Esteban laboratory for their support, and J. Chen, Y. Chen, S. Chu, F. Cui, S. Huang, D. P. Ibáñez, K. Lai, R. Luo, X. Qing and F. Zhang for technical assistance. We also thank A. Schambach from the Department of Experimental Hematology, Hannover Medical School for providing the polycistronic 4F-dT lentiviral vector, Y. Chen from Tsinghua University for providing the Atg5 KO mice (with permission from N. Mizushima and RIKEN BioResource Center) and Q. Chen from the Institute of Zoology, Chinese Academy of Sciences. This work was financially supported by the National Basic Research Program of China (973 Program of China, 2011CBA01106, 2011CBA01004, 2011CB965201 and 2012CB966802), the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (XDA01020106, XDA01020202 and XDA01020401), the National Natural Science Foundation of China (31301057, 31421004, 91213304 and 31371513), the Ministry of Science and Technology International Technology Cooperation Program (2012DFH30050) and a joint German–Chinese grant from the German Academic Exchange Service (DAAD), the German Ministry of Research and the Ministry of Science and Technology of China to D.P. and A. Schambach. H-F.T. is financially supported by the Theme Based Research Scheme (T12-705/11) and the Innovation and Technology Support Programme (Tier 3) (ITS/303/12).
Author information
Authors and Affiliations
Contributions
B.Q., M.A.E. and D.P. conceived the idea. B.Q. supervised the study with help from D.P. and M.A.E. Y.W. and Y.L. conducted most of the experiments. Y.W., Y.L. and B.Q. analysed the data. H.Z., Y.H., P.Z., Y.T., X.Q., Y.Y., S.N., M.Z., L.L., Y.X., Q.Z., Z.L., C.B. and H.S. contributed to the experiments. W.L. contributed to electron microscopy. L.L., H-F.T., X.B. and M.A.E. provided infrastructural support. X.L., B.L. and W-Y.C. provided relevant advice. B.Q., M.A.E. and D.P. wrote the manuscript. D.P. approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
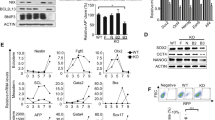
Supplementary Figure 4 mRNA expression in different reprogramming systems and autophagy induction using the polycistronic lentivirus and the 2° system.
(a) qPCR analysis of the 4F mRNA level in retroviral reprogramming relative to Ctrl day 6. Means ± s.e.m. from n = 3 independent experiments with 3 technical replicates are shown. (b) Phase contrast and fluorescence of Oct4-GFP (OG2) ESCs in 2i+LIF on gelatin (ESCs 2i), serum+LIF on feeder (ESCs serum) and differentiation (withdrawal of 2i and LIF for 3 days, Diff. D3). Scale bars: 100 μm. (c) Western blotting for LC3B, p62 and ACTIN in MEFs, mouse ESCs in 2i+LIF and serum+LIF medium. Low (L) and high (H) cell density indicate 2- or 3-day culture respectively. (d) Quantification of LC3B-II relative to ACTIN in c. (e) qPCR analysis of Oct4 and Nanog in cells after 3-day differentiation (right panel in b) relative to ESCs in 2i+LIF. Data shown are the mean of 3 replicates and are from 1 experiment. (f) Western blotting for LC3B, p62 and ACTIN in MEFs, mouse ESCs in 2i+LIF and 3-day differentiation. (g) Quantification of LC3B-II relative to ACTIN in (f). (h) qPCR analysis for the 4F in the 4F-dT system on day 6 (Ctrl, 1XLenti-Flag). Data shown are the mean of 3 replicates and are from 1 of 2 representative experiments. (i) Western blotting for LC3B and ACTIN in MEFs transduced with Ctrl or 4F-dT on day 3, 5 and 7 post infection. (j) qPCR analysis for 4F in the 2° system relative to Ctrl (-DOX). Data shown are the mean of 3 replicates and are from 1 of 2 representative experiments. (k) Western blotting for LC3B and ACTIN in the 2° system, −/+Baf. Uncropped images of blots are shown in Supplementary Fig. 6. Source data are provided in Supplementary Table 4.
Supplementary Figure 5 The Yamanaka factors regulate autophagy differently independent of p53.
(a) qPCR for a large panel of Atg genes in MEFs transduced with 4F retroviruses at different time points and ESCs. (b) Western blotting for Atg genes in MEFs and ESCs. (c) qPCR analysis of the knockdown efficiency of combined shRNAs for Atg5, Beclin1 and Vps34 on 4F day 5. Only 1 control of shLuc is shown. (d) Western blotting for LC3B in MEFs transduced with combined factors on day 1, 3 and 5, treated with Baf. Ctrl, 2XFlag Numbers indicate quantification of LC3B-II relative to ACTIN. (e) qPCR for indicated genes in p53 KO TTFs transduced with 4F. Means ± s.e.m. from n = 3 independent experiments with 3 technical replicates are shown. (f) qPCR for the indicated genes in p53 KO TTFs transduced with 2 factors. Ctrl, 2XFlag. (g) Western blotting for LC3B and ACTIN in MEFs transduced with Ctrl (4XFlag) or 4F in iCD1+BMP4 medium under −/+Baf conditions. The samples were from one experiment and run on the same gel. The additional bands were sliced out. A solid line was put to separate the two slice parts. (h) Western blotting for LC3B and ACTIN in MEFs transduced with Ctrl (1XLenti-Flag) or 3F-dT, −/+Baf. Data shown are the mean of 3 replicates and are from 1 of 2 representative experiments (a,f). Source data are provided in Supplementary Table 4. Uncropped images of blots are shown in Supplementary Fig. 6.
Supplementary Figure 6 Autophagy impairs 4F reprogramming.
(a) Apoptosis analysis of 4F and Atg shRNA transduced cells. (b) Western blotting for the indicated genes to confirm the expression of Atg gene WT and dominant-negative (DN) mutants in transduced MEFs. (c) Western blotting for LC3B in MEFs transduced with 4F and DN mutants on day 5 (−/+Baf). (d) Effect of the above mutants on reprogramming. (e) Western blotting for LC3B in MEFs transduced with 4F and the indicated WT genes on day 5 (−/+Baf). (f) Effect of these WT genes on reprogramming. (g) FACS analysis of dTomato-positive cells in 4F-dT transduced MEFs from Atg5 WT or KO embryos. Data are plotted from 1 experiment with 3 technical replicates. (h) Western blotting for exogenous SOX2 in 4F-dT transduced MEFs from Atg5 WT and KO embryos. (i) Representative images of an iPSC clone (SSEA1+/dTomato-, arrow) and a non-iPSC clone (SSEA1-/dTomato +, dash circle). Scale bar: 100 μm. (j) Apoptosis analysis in Atg5 KO MEFs transduced with Ctrl or Atg5. Means ± s.e.m. from n = 3 independent experiments with 3 technical replicates are shown. (k) Effect of Atg5 rescue on reprogramming in 4F transduced OG2-Atg5 KO MEFs. (l) Atg5 rescue has no effect on reprogramming efficiency in 3F-dT transduced Atg5 KO MEFs. Data shown are the mean of 3 replicates and are from 1 of 2 (a), 3 (d,f,l) and 5 (k) representative experiments. Source data are provided in Supplementary Table 4. Uncropped images of blots are shown in Supplementary Fig. 6.
Supplementary Figure 7 Characterization of iPSC clones.
(a) Genomic PCR analysis of transgene integration of 3 representative iPSC clones generated with retroviral 4F and shBeclin1, shVps34 and Atg16l-M. Negative indicates ESCs; positive indicates Actin. (b) Phase contrast, fluorescence and immunofluorescence microscopy of representative iPSC clones. Scale bars: 100 μm. (c) Bright-field view of Atg5 KO 4F iPSC clone C1. Scale bar: 100 μm. (d) Western blotting for LC3B and ATG5-12 in Atg5 KO-4F C1. (e) qPCR analysis of the expression of endogenous pluripotent genes and the silencing of 4F transgenes in iPSCs. Data are plotted from 1 experiment with 3 technical replicates. (f) DNA methylation profile of the Oct4 and Nanog proximal promoters in the indicated iPSCs, ESCs and MEFs. (g) Karyotype analysis of these iPSCs. (h) Hematoxylin/eosin staining of the teratomas formed from the shRNA iPSCs. Scale bar: 100 μm. (i) Chimeric mice produced with the above clones and germline transmission using Atg16l-M-C2 iPSCs (F1). Source data are provided in Supplementary Table 4. Uncropped images of blots are shown in Supplementary Fig. 6.
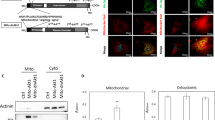
Supplementary Figure 8 Inhibition of mTORC1 mediates mitochondrial remodelling.
(a) Western blotting for MTOR and ATK-mTORC1 signalling in early reprogramming of 2° system (+ DOX). (b) Western blotting for mTORC1 activity in MEFs and ESCs. (c) Western blotting shows the change of mTORC1 signalling pathway in p53 KO TTFs in early reprogramming. Ctrl, 4XFlag. (d) qPCR analysis for Mtor in MEFs transduced with indicated factors on day 1. (e) Western blotting for MTOR, LC3B and p62 in MEFs transduced with the indicated factors on day 1 (−/+Baf). (f) qPCR analysis of genes responsible for mitochondrial biogenesis. (g) FACS analysis shows the effect of Tsc2 shRNAs on cell size. (h) qPCR analysis to confirm the expression of the indicated genes in 4F reprogramming on day 5. Data are plotted from 1 experiment with 3 technical replicates. (i) qPCR analysis of genes responsible for mitochondrial biogenesis in MEFs transduced with 4F and the indicated genes on day 5. (j) FACS analysis for cell size in MEFs transduced with indicated genes on day 5. (k) FACS analysis cell proliferation in MEFs transduced with indicated genes on day 5. Data shown are the mean of 3 replicates and are from 1 of 2 representative experiments (d,k). Means ± s.e.m. from n = 3 (f,g,i) and 4 (j) independent experiments with 3 technical replicates are shown. Source data are provided in Supplementary Table 4. Uncropped images of blots are shown in Supplementary Fig. 6.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3556 kb)
Supplementary Table 1
Supplementary Information (XLSX 48 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Li, Y., Zhang, H. et al. Autophagy and mTORC1 regulate the stochastic phase of somatic cell reprogramming. Nat Cell Biol 17, 715–725 (2015). https://doi.org/10.1038/ncb3172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3172
This article is cited by
-
A fast chemical reprogramming system promotes cell identity transition through a diapause-like state
Nature Cell Biology (2023)
-
BNIP3 (BCL2 interacting protein 3) regulates pluripotency by modulating mitochondrial homeostasis via mitophagy
Cell Death & Disease (2022)
-
Protein quality control of cell stemness
Cell Regeneration (2020)
-
Functional characterization of SOX2 as an anticancer target
Signal Transduction and Targeted Therapy (2020)
-
Notch pathway: a bistable inducer of biological noise?
Cell Communication and Signaling (2019)