Abstract
YAP (Yes-associated protein) is a transcription co-activator in the Hippo tumour suppressor pathway and controls cell growth, tissue homeostasis and organ size. YAP is inhibited by the kinase Lats, which phosphorylates YAP to induce its cytoplasmic localization and proteasomal degradation. YAP induces gene expression by binding to the TEAD family transcription factors. Dysregulation of the Hippo–YAP pathway is frequently observed in human cancers. Here we show that cellular energy stress induces YAP phosphorylation, in part due to AMPK-dependent Lats activation, thereby inhibiting YAP activity. Moreover, AMPK directly phosphorylates YAP Ser 94, a residue essential for the interaction with TEAD, thus disrupting the YAP–TEAD interaction. AMPK-induced YAP inhibition can suppress oncogenic transformation of Lats-null cells with high YAP activity. Our study establishes a molecular mechanism and functional significance of AMPK in linking cellular energy status to the Hippo–YAP pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
14 April 2015
In the Methods section 'Antibodies and reagents' there was a typographical error in the company name GeneTex. The sentence should have read 'Anti-phosphorylated Ser 94 antibody was generated by immunizing rabbits with phosphopeptides (Abbiotec and GeneTex, 1:500)'. This has now been corrected online.
References
Oh, H. & Irvine, K. D. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 20, 410–417 (2010).
Pan, D. The Hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Zhao, B., Tumaneng, K. & Guan, K. L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 (2011).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Hao, Y., Chun, A., Cheung, K., Rashidi, B. & Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283, 5496–5509 (2008).
Zhao, B., Li, L., Tumaneng, K., Wang, C. Y. & Guan, K. L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ−TRCP. Genes Dev. 24, 72–85 (2010).
Liu, C. Y. et al. The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{β}−TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 (2010).
Lu, L. et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl Acad. Sci. USA 107, 1437–1442 (2010).
Cai, J. et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388 (2010).
Lee, K. P. et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. USA 107, 8248–8253 (2010).
Ota, M. & Sasaki, H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135, 4059–4069 (2008).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014).
Strano, S. et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 18, 447–459 (2005).
Lapi, E. et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol. Cell 32, 803–814 (2008).
Cottini, F. et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat. Med. 20, 599–606 (2014).
Schlegelmilch, K. et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 (2011).
Zhao, B. et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 (2012).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Mo, J. S., Yu, F. X., Gong, R., Brown, J. H. & Guan, K. L. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 26, 2138–2143 (2012).
Miller, E. et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 19, 955–962 (2012).
Yu, F. X. et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 27, 1223–1232 (2013).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011).
Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 (2011).
Hardie, D. G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908 (2011).
Vassilev, A., Kaneko, K. J., Shu, H., Zhao, Y. & DePamphilis, M. L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229–1241 (2001).
Mahoney, W. M. Jr, Hong, J. H., Yaffe, M. B. & Farrance, I. K. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem. J. 388, 217–225 (2005).
Tian, W., Yu, J., Tomchick, D. R., Pan, D. & Luo, X. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc. Natl Acad. Sci. USA 107, 7293–7298 (2010).
Chan, E. H. et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 (2005).
Praskova, M., Xia, F. & Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311–321 (2008).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Stambolic, V., Woodgett, J. R., Fantus, I. G., Pritchard, K. I. & Goodwin, P. J. Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Res. Treat. 114, 387–389 (2009).
Moreno, D., Knecht, E., Viollet, B. & Sanz, P. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett. 582, 2650–2654 (2008).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009).
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 (2014).
Kim, M. et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 32, 1543–1555 (2013).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Budanov, A. V. & Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460 (2008).
Li, Z. et al. Structural insights into the YAP and TEAD complex. Genes Dev. 24, 235–240 (2010).
Chen, L. et al. Structural basis of YAP recognition by TEAD4 in the Hippo pathway. Genes Dev. 24, 290–300 (2010).
Yu, F. X. & Guan, K. L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013).
DeRan, M. et al. Energy stress regulates Hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 9, 495–503 (2014).
Zhang, H. et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J. Biological Chem. 284, 13355–13362 (2009).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, http://dx.doi.org/10.1038/ncb3113 (2015).
Acknowledgements
We would like to thank A. Hong and F. Flores for critical reading of the manuscript. This work was supported by grants from National Institutes of Health (CA132809, EY022611, DEO15964 and CA23100, K-L.G.). C.G.H. is supported by a Postdoctoral Fellowship from the Danish Council for Independent Research, Natural Sciences.
Author information
Authors and Affiliations
Contributions
J-S.M. and K-L.G. designed the experiments, analysed data and wrote the paper. J-S.M. performed the experiments with assistance from Z.M., Y.C.K., H.W.P., C.G.H. and S.K.; D-S.L. established the Lats knockout MEFs. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
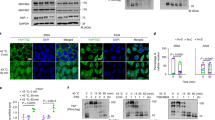
Supplementary Figure 1 Energy stress induces YAP phosphorylation.
(a) Osmotic stress does not affect YAP and TAZ mobility shift. HEK293A cells were treated with different concentrations of 2-DG or sorbitol for 1 h. Cells were then lysed and subjected to immunoblotting using the indicated antibodies. (b) 2-DG induces YAP phosphorylation. HEK293A cells were treated with different concentrations of 2-DG for 30 min. Cells were then lysed and cell lysates were subjected to immunoblotting using the indicated antibodies. (c) Experiments are the same as in panel an exception cells were treated with 25 mM 2-DG for various times. (d–f) Effect of 2-DG on YAP phosphorylation in different cell lines. C2C12 cells were treated with 10 mM and 25 mM 2-DG (d); MCF10A cells were treated with 10 and 25 mM 2-DG (e); HeLa cells were treated with 10 and 25 mM (f). (g) Glucose starvation inhibits YAP and TEAD1 interaction. HEK293A cells were starved with glucose for 2 h as indicated. Endogenous YAP/TAZ and the co-immunoprecipitated TEAD were detected by western blot.
Supplementary Figure 2 2-DG does not affect MST phosphorylation.
(a) Effect of MST overexpression on 2-DG induced YAP phosphorylation. HEK293A cells were transiently co-transfected with indicated plasmids. After transfection, cells were treated with 25 mM 2-DG for 1 h and YAP phosphorylation status was determined by phos-tag gel. (b) 2-DG does not affect MST1 phosphorylation. Endogenous MST1 was immunoprecipitated from control or 2-DG (25 mM, 2 h) treated HEK293A cells. MST1 phosphorylation was detected by a phospho-MST (T183) specific antibody.
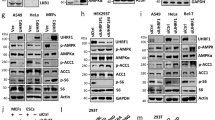
Supplementary Figure 3 Activation of AMPK increases YAP phosphorylation in various cell lines.
(a) Genotyping of AMPKa1 and AMPKa2 wild-type (WT) and mutant (KO) alleles. DNA was isolated from AMPK+/+ or AMPK−/− cells as indicated. Genotyping was performed by PCR using primers specific to wild-type AMPKa (left side of the dash line) or AMPKa KO (right side of the dash line). The PCR products were run on the same agarose gel. Samples in the left panel are PCR products using primer specific to AMPKa1 where samples in the right panel are PCR products using primers specific to AMPKa2. (b) Cells were treated with 25 mM 2-DG for various times as indicated and lysed. Lysates were incubated with lambda phosphatase (λ PPase) as indicated for 1 h. Endogenous YAP mobility was examined by western blot. (c) AICAR increases YAP phosphorylation in hepatocytes. Primary mouse hepatocytes were treated with different doses of AICAR for 8 h. Cell lysates were used for immunoblotting with indicated antibodies. (d) HepG2 cells were treated with different doses of AICAR for 2 h. (e) HepG2 cells were treated with different doses of A769662 for 2 h.
Supplementary Figure 4 Identification of AMPK phosphorylation sites in YAP.
(a) AMPK induces mobility shift of YAP-5SA. GST-YAP 5SA was used as substrates for in vitro AMPK phosphorylation. GST- YAP mobility was examined by western blot (b) Mass spectrometry analyses of AMPK-phosphorylated YAP. GST-YAP-5SA was purified from E.coli and was used as a substrate for an in vitro AMPK assay. After electrophoresis, the phosphorylated GST-YAP 5SA band was cut and digested with trypsin/thermolysine followed by mass spectrometry analyses. The green coloured bars (top) and amino acid residues (lower part) are sequences that were detected by mass spectrometry. Phosphorylation sites identified by mass spectrometry are indicated on top of the amino acid sequence. (c) S94 is the major AMPK phosphorylation site in the YAP (51-121) fragment. Experiments were similar to panel a. (d) All three indicated residues of YAP were mutated to alanine. A luciferase reporter controlled by multiple TEAD binding sequences was transfected into HEK293T cells together with 5 × UAS-luciferase reporter for Gal4-TEAD4 and Renilla constructs and indicated plasmids. After 48 h, the firefly luciferase activity was measured and normalized to the co-transfected Renilla luciferase internal control. (d) Two different substrates of AMPK, TSC2 and ULK1, and YAP truncations were expressed, purified, and used as substrates for in vitro AMPK phosphorylation in the presence of 32P-ATP Phosphorylation was detected by autoradiography. GST-ULK1 (279-425), TSC2 (1300-1367) and YAP fragment proteins were detected by Coomassie staining. (e) Experiments are same as in panel c. except that a GST-YAP truncated form and UKL1 were used as substrates for in vitro AMPK phosphorylation in the presence of 32P-ATP for the indicated times. (f) Experiments are the same as in panel d. exception GST-YAP WT and UKL1 used as substrate for the indicated times. (g) AMPK has no effect on the YAP and RUNX2 interaction. HEK293A cells were transfected with the indicated plasmids. Flag-RUNX2 was immunoprecipitated and the co-precipitated HA-YAP was detected by Western blot.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1808 kb)
Rights and permissions
About this article
Cite this article
Mo, JS., Meng, Z., Kim, Y. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 17, 500–510 (2015). https://doi.org/10.1038/ncb3111
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3111
This article is cited by
-
Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury
Cell Death & Disease (2024)
-
AMPK stimulation inhibits YAP/TAZ signaling to ameliorate hepatic fibrosis
Scientific Reports (2024)
-
The LKB1–TSSK1B axis controls YAP phosphorylation to regulate the Hippo–YAP pathway
Cell Death & Disease (2024)
-
Targeting FLT3-TAZ signaling to suppress drug resistance in blast phase chronic myeloid leukemia
Molecular Cancer (2023)
-
Molecular mechanisms of AMPK/YAP/NLRP3 signaling pathway affecting the occurrence and development of ankylosing spondylitis
Journal of Orthopaedic Surgery and Research (2023)