Abstract
The contractile actomyosin cytoskeleton and its connection to the plasma membrane are critical for control of cell shape and migration. We identify three STRIPAK complex components, FAM40A, FAM40B and STRN3, as regulators of the actomyosin cortex. We show that FAM40A negatively regulates the MST3 and MST4 kinases, which promote the co-localization of the contractile actomyosin machinery with the Ezrin/Radixin/Moesin family proteins by phosphorylating the inhibitors of PPP1CB, PPP1R14A–D. Using computational modelling, in vitro cell migration assays and in vivo breast cancer metastasis assays we demonstrate that co-localization of contractile activity and actin–plasma membrane linkage reduces cell speed on planar surfaces, but favours migration in confined environments similar to those observed in vivo. We further show that FAM40B mutations found in human tumours uncouple it from PP2A and enable it to drive a contractile phenotype, which may underlie its role in human cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 (2009).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Keren, K., Yam, P. T., Kinkhabwala, A., Mogilner, A. & Theriot, J. A. Intracellular fluid flow in rapidly moving cells. Nat. Cell Biol. 11, 1219–1224 (2009).
Charras, G. & Paluch, E. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730–736 (2008).
Lammermann, T. & Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009).
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S. & Sahai, E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006).
Sahai, E. & Marshall, C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5, 711–719 (2003).
Sanz-Moreno, V. & Marshall, C. J. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr. Opin. Cell Biol. 22, 690–696 (2010).
Madsen, C. D. & Sahai, E. Cancer dissemination—lessons from leukocytes. Dev. Cell 19, 13–26 (2010).
Friedl, P. & Wolf, K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 (2010).
Sanz-Moreno, V. et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523 (2008).
Matsumura, F. & Hartshorne, D. J. Myosin phosphatase target subunit: many roles in cell function. Biochem. Biophys. Res. Commun. 369, 149–156 (2008).
Hartshorne, D. J., Ito, M. & Erdodi, F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J. Biol. Chem. 279, 37211–37214 (2004).
Grassie, M. E., Moffat, L. D., Walsh, M. P. & MacDonald, J. A. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 510, 147–159 (2011).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Fehon, R. G., McClatchey, A. I. & Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 (2010).
Fukata, Y. et al. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 141, 409–418 (1998).
Janssens, V. & Goris, J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439 (2001).
Basu, S. PP2A in the regulation of cell motility and invasion. Curr. Protein Pept. Sci. 12, 3–11 (2011).
Butler, T., Paul, J., Europe-Finner, N., Smith, R. & Chan, E. C. Role of serine-threonine phosphoprotein phosphatases in smooth muscle contractility. Am. J. Physiol. Cell Physiol. 304, C485-504 (2013).
Bai, S. W. et al. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 9, 54 (2011).
Chen, H. W. et al. CKA, a novel multidomain protein, regulates the JUN N-terminal kinase signal transduction pathway in Drosophila. Mol. Cell. Biol. 22, 1792–1803 (2002).
Goudreault, M. et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteomics 8, 157–171 (2009).
Glatter, T., Wepf, A., Aebersold, R. & Gstaiger, M. An integrated workflow for charting the human interaction proteome: insights into the PP2A system. Mol. Syst. Biol. 5, 237 (2009).
Ribeiro, P. S. et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521–534 (2010).
Herzog, F. et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science 337, 1348–1352 (2012).
Hwang, J. & Pallas, D. C. STRIPAK complexes: structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 47, 118–148 (2014).
Preisinger, C. et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J. Cell Biol. 164, 1009–1020 (2004).
Lu, T. J. et al. Inhibition of cell migration by autophosphorylated mammalian sterile 20-like kinase 3 (MST3) involves paxillin and protein-tyrosine phosphatase-PEST. J. Biol. Chem. 281, 38405–38417 (2006).
Matsuki, T. et al. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell 143, 826–836 (2010).
Ten Klooster, J. P. et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 (2009).
Gloerich, M. et al. Rap2A links intestinal cell polarity to brush border formation. Nat. Cell Biol. 14, 793–801 (2012).
Sugden, P. H., McGuffin, L. J. & Clerk, A. SOcK, MiSTs, MASK and STicKs: the GCKIII (germinal centre kinase III) kinases and their heterologous protein-protein interactions. Biochem. J. 454, 13–30 (2013).
Montell, D. J., Rorth, P. & Spradling, A. C. Slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71, 51–62 (1992).
Montell, D. J. Border-cell migration: the race is on. Nat. Rev. Mol. Cell Biol. 4, 13–24 (2003).
Montell, D. J., Yoon, W. H. & Starz-Gaiano, M. Group choreography: mechanisms orchestrating the collective movement of border cells. Nat. Rev. Mol. Cell Biol. 13, 631–645 (2012).
Silver, D. L. & Montell, D. J. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell 107, 831–841 (2001).
Duchek, P. & Rorth, P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science 291, 131–133 (2001).
Duchek, P., Somogyi, K., Jekely, G., Beccari, S. & Rorth, P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17–26 (2001).
Somogyi, K. & Rorth, P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev. Cell 7, 85–93 (2004).
Kean, M. J. et al. Structure-function analysis of core STRIPAK proteins: a signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065–25075 (2011).
Amano, M., Nakayama, M. & Kaibuchi, K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67, 545–554 (2010).
Croft, D. R. et al. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 64, 8994–9001 (2004).
Tozluoglu, M. et al. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 15, 751–762 (2013).
Gyorffy, B., Lanczky, A. & Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer 19, 197–208 (2012).
Fidalgo, M. et al. Adaptor protein cerebral cavernous malformation 3 (CCM3) mediates phosphorylation of the cytoskeletal proteins ezrin/radixin/moesin by mammalian Ste20-4 to protect cells from oxidative stress. J. Biol. Chem. 287, 11556–11565 (2012).
Eto, M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J. Biol. Chem. 284, 35273–35277 (2009).
Wolf, K. et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267–277 (2003).
Carragher, N. O. et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene 25, 5726–5740 (2006).
Voss, K. et al. Functional analyses of human and zebrafish 18-amino acid in-frame deletion pave the way for domain mapping of the cerebral cavernous malformation 3 protein. Hum. Mutat. 30, 1003–1011 (2009).
Zheng, X. et al. CCM3 signaling through sterile 20-like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J. Clin. Invest. 120, 2795–2804 (2010).
Faurobert, E. et al. CCM1-ICAP-1 complex controls β1 integrin-dependent endothelial contractility and fibronectin remodeling. J. Cell Biol. 202, 545–561 (2013).
Davoli, T. et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 155, 948–962 (2013).
Lorentzen, A., Bamber, J., Sadok, A., Elson-Schwab, I. & Marshall, C. J. An ezrin-rich, rigid uropod-like structure directs movement of amoeboid blebbing cells. J. Cell Sci. 124, 1256–1267 (2011).
Petrie, R. J., Koo, H. & Yamada, K. M. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Hirata, E. et al. In vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J. Cell Sci. 125, 858–868 (2012).
Yeung, T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 (2005).
Gyorffy, B. et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 123, 725–731 (2010).
Ringner, M., Fredlund, E., Hakkinen, J., Borg, A. & Staaf, J. GOBO: gene expression-based outcome for breast cancer online. PLoS ONE 6, e17911 (2011).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Acknowledgements
C.D.M., S.H., M.T., A.B., G.F., P.A.B., B.T. and E.S. are financially supported by Cancer Research UK. C.D.M. was further supported by a FEBS long-term fellowship. We thank laboratory members for help and advice throughout this work. We thank N. O’Reilly and S. Kjaer for help with peptide synthesis and protein purification, and members of the BRU for help with metastasis assays.
Author information
Authors and Affiliations
Contributions
C.D.M. and E.S. carried out all experiments except those noted otherwise. S.H. performed all kinase assays and immunoprecipitation assays. G.F. and B.T. performed the fly screen. M.T. and P.A.B. made the mathematical model. A.B. helped with structured illumination microscopy. C.D.M. and E.S. conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 RNAi screen in the egg chamber of Drosophila melanogaster and in human A431 carcinoma cells.
RNAi screen targeting genes that are required for border cell migration and actin organization in follicle epithelial cells within the Drosophila egg chamber. (a) Schematic view of the screening strategy. (b) Flow diagram of the screen. siRNA lines crossed on to the hh.GAL4 driver were assumed to have no strong phenotype within the egg chamber (either border cell or follicular epithelium) if the flies were healthy and viable—these were not further analysed. The 1393 lethal siRNA lines were further analysed for egg chamber phenotypes by crossing the Gr1.GAL4 driver—594 of these lines were fertile and therefore not analysed further. The remaining 844 lines were analysed in more detail. (c) Cell morphologies observed on siRNA depletion in human A431 carcinoma cells. A431 cells were transfected with the indicated siRNAs and plated on collagen-I/matrigels. (n = 3 screens). The cells were fixed and stained with Phalloidin-TRITC (red) and DAPI (blue) 72 h post-transfection. Only genes affecting the cell morphology are shown. (d) Border cell migration delay in the CG11526 (FAM40) and CKA (STRN) RNAi flies. Representative images of the Drosophila melanogaster egg chamber at stage 9 and 10. The egg chambers were stained for the basolateral marker Discs Large (Dlg, green) and DAPI (magenta). RNAi expression was driven in border cells using a Gr1-Gal4 driver. (e) Quantification of border cell clusters that reached their destination at stage 9 (upper histogram, n = animals; Ctr, 43; FAM40ARNAi, 23; CKARNAi, 28). Estimation of the distance travelled by the border cell cluster at stage 10 (lower histogram, n = animals; Ctr, 64; FAM40ARNAi, 47; CKARNAi, 43): 100%—reached the oocyte, >50%—travelled more than half the distance toward the oocyte; >25%—travelled more than one forth the distance toward the oocyte; 0%—did not leave the anterior part of the egg chamber. (f) Representative images of the egg chamber at stage 9 stained for with DAPI (cyan) and pMoesin (red, upper panels) or pMyosin2 (red, lower panels). The insets represent a zoomed area. Scale bars, 10 μm, unless indicated otherwise.
Supplementary Figure 2 Epithelial organisation on FAM40A, FAM40B and STRN3 depletion in human cancer cells.
(a,b) siRNA depleted A431 cells plated on collagen-I/matrigels. Cells were fixed 72 h post-transfection and stained (a) against pS19-MLC (green) and (b) pT18S19-MLC (blue) and E-Cadherin (green). F-actin is stained with Phalloidin-TRITC (red). (c) siRNA knockdown efficiency +/s.e.m of FAM40A and FAM40B mRNA in A431 cells. Immunoblotting analysis of FAM40B protein levels on FAM40B depletion. (d) Deconvolution of siRNA oligos in A431 cells plated on collagen-I/matrigel. Cells were stained 72 h post-transfection against pS19-MLC2 (white) and F-actin (red). Quantification of A431 cell area +/−s.e.m (upper histogram, n = fields of cells; Ctr, 19; siFAM40A_2, 9; siFAM40A_5, 6; siFAM40A_7, 5; siFAM40B-3, 12; siFAM40B-6, 6; siFAM40B-7, 7) and mean fluorescence pS19-MLC2 intensities +/−s.e.m (lower histogram, n = fields of cells; Ctr, 10; siFAM40A_2, 6; siFAM40A_5, 4; siFAM40A_7, 4; siFAM40B-3, 6; siFAM40B-6, 6; siFAM40B-7,6). (e) Quantification of mean fluorescence cortical intensities of pS19-MLC2 (left panel, n = cells; Ctr, 33; siFAM40A, 46; siFAM40B, 47) and pERM (right panel, n = cells; Ctr, 29; siFAM40A, 35; siFAM40B, 43) in MDA-MB-231 cells. Box and whiskers graph: Line = Median, Box = distribution of 50% of values, whiskers = min. to max. (f) siRNA depleted MDA-MB-231 cells were fixed 72 h post-transfection. Focal adhesions were stained with phospho-tyrosine antibody (cl. 4G10), and thin sections of the focal adhesions in the basal plasma membrane were acquired using confocal microscopy. Morphometric analysis was performed on individual cells. Two independent experiments were conducted and 20–25 cells quantified. n = focal adhesions; Ctr, 468; siFAM40A, 401; siFAM40B, 558). (g,h) Cell surface expression of integrin β1 (ITGB1) using flow cytometry was performed on MDA-MB-231 cells 72 h post-transfection. Cell surface expression of ITGB1 +/−s.d. was detected using the HUTS-21 antibody detecting the active form with or without the presence of 0.5 mM MnCl2 to activate all integrins on the cell surface (n = 3 experiments). (i) Quantification of pS19-MLC2 and cytosolic GFP co-localisation + /s.e.m. (n = cells; siCtr, 25; siFAM40A, 16; siFAM40B, 17). (j) siRNA depleted MDA-MB-231-Ezrin-GFP cells were fixed 72 h post-transfection. Cells were stained for MyoIIA (red), and thin 150 nm sections were acquired using Structured Illumination Microscopy (left panels). The spatial correlation (r) between Ezrin-GFP (green) and MyoIIA (red) was then determined on FAM40A and FAM40B depletion and compared to control cells (right panels). Statistical test were performed using 1way ANOVA, Sidak’s multiple comparison test, ∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001. All experiments were conducted at least 3 independent times. Scale bars, 10 μm, unless indicated otherwise.
Supplementary Figure 3 Epithelial organization on MST1, MST2, MST3, MST4, MST3&4, SOK1 and CCM3 depletion in A431 cells.
(a,b) The human genes, MST3, MST4, MST3&4 and CCM3, were depleted in A431 cells using siRNA smart pools and plated on collagen-I/matrigels. The cells were fixed 72 h post-transfection and stained against pS19-MLC (white) and F-actin (red). (c) Immunoblotting analysis shows the knockdown efficiency of MST3 and MST4 proteins on siRNA depletion. (d) Quantification of A431 cell area +/−s.e.m on siRNA depletion and plating on collagen-I/matrigels (n = fields of cells; siCtr, 17; siMST3, 8; siMST4, 6; siMST3&4, 13; siCCM3, 13). (e) Deconvolution of three SOK1 siRNA oligos in MDA-MB-231 cells plated on collagen-I/matrigel. Cells were stained 72 h post-transfection against pS19-MLC2 (white) and F-actin (red). (f) Knockdown efficiency of SOK1 mRNA using three individual siRNA oligos as compared to control cells. (g) Double depletion of MST1&2 in MDA-MB-231 cells using siRNA smart pools. The cells were fixed 72 h post-transfection and stained against pS19-MLC (white) and F-actin (red). (h) Quantification of MDA-MB-231 cell area +/−s.e.m on siRNA depletions (n = fields of cells; siCtr, 19; siMST1, 5; siMST2, 5; siMST1&2, 8; siMST3, 2; siMST4, 2; siMST3&4, 14; siSOK, 2; siMST3&4&SOK, 2). (i) Double depletion using smart pools of FAM40A or FAM40B with either ITGB1, talin (TLN) or vinculin (VCL). MDA-MD231 cells were stained 72 h post-transfection against pS19-MLC2 (green) and F-actin (red). (j) Epistasis experiments in MDA-MB-231 (left panel) and A431 cells (right panel) show that MST3&4 depletion rescues the contractile phenotype observed on Okadaic acid treatment. The cells were fixed 72 h post-transfection and stained against pS19-MLC (green) and F-actin (red). All statistical test were performed using 1way ANOVA, Sidak’s multiple comparison test, ∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001. All experiments were conducted at least 3 independent times. Scale bars, 10 μm, unless indicated otherwise.
Supplementary Figure 4 Temporal regulation of MLC and Ezrin.
(a,b) Serum stimulation of serum starved A431-MLC-GFP (a) and A431-Ezrin-GFP cells (b) induces an immediate translocation of MLC-GFP and Ezrin-GFP to the actomyosin network at the outer edge (white arrows) of A431 cell clusters and at cell-cell junctions (red arrows). Quantification of the serum-induced MLC-GFP and Ezrin-GFP translocation +/−s.e.m was performed by normalizing the mean fluorescence intensity at cell border (left graph) and cell-cell junctions (right graph) to the mean fluorescence intensity of the adjacent cytosolic fraction. n = regions of individual measurements (63x) at edge of cluster and cell-cell contacts; MLC, 70; Ez, 35. The values are pooled from three independent experiments. (c) F-actin staining of serum starved A431 cells and after 5 minutes of FCS stimulation. FCS stimulation induces slight blebbing at the cortex of the cells. Blebs are indicated by white arrows. All experiments were conducted at least 3 independent times. Scale bars, 10 μm.
Supplementary Figure 5 Spatial localisation of MLC and Ezrin during migration.
(a) Time lapse analysis of the temporal and spatial localisation of MLC-Cherry (red) and Ezrin-GFP (green) in MDA-MB-231 cells (left image). White arrow indicates direction of migration. Quantification of the spatial distribution of MLC-Cherry and Ezrin-GFP intensities +/−s.e.m. (n = 14 cells). Unpaired student’s t-test (two-tailed), ∗∗∗P < 0.001. Left histogram shows MLC-Cherry in the front and rear of the cells. Right histogram shows the Ezrin-GFP in the front and rear of the cells. (b) 2D migration analysis of siRNA transfected MDA-MB-231 cells stably expressing either H2b-GFP were generated using MetaMorph Software. Motion analysis and migration tracks were generated from H2b-GFP images taken over 12 h at 5 min intervals. Analysis of 2D migration persistency using Mathematica Software. Box and Whiskers graph: Line = Median, Box = distribution of 50% of values, Whiskers = 20–80 percentile. n = fields of cells (10×); (siCtr, 34; siFAM40A, 37; siFAM40B, 30; siMST3&4, 35; siCCM3, 32). (c) Schematics of the computational model to investigate the effect of perturbing the co-localisation of actomyosin contraction and actin cortex to plasma membrane attachment during migration through channels that are 8 μm wide and 6.5 μm long. These dimensions correspond to those of commercially available transwell chambers. The model predicts that co-localisation favours migration through 8 μm channels. All statistical test were performed using 1way ANOVA, Sidak’s multiple comparison test, unless stated otherwise, ∗∗P < 0.01,∗∗∗P < 0.001. All experiments were conducted at least 3 independent times. Scale bar, 10 μm.
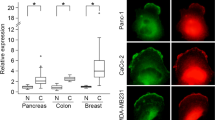
Supplementary Figure 6 Clinical significance of MST3, MST4 and CCM3 expression in breast cancer patients.
(a,b) Immunoblotting analyses demonstrating the levels of endogenous MST3 and FAM40A (grey arrows) and exogenously expressed MST3-GFP and FAM40A-GFP (black arrows) in A431 (a) and MDA-MB-231 cells (b). (c) Analysis of publicly available datasets show the mRNA log 2 expression levels of MST3, MST4, CCM3, STRN3 and STRN4 observed in human breast cancer patients (n = 1881 patients). The statistical analysis was based on human cancer sub-types (left panels), ER-status (middle panels), and disease grade (right panels). The box plots are made with default parameters to the box plot function in R. Box and Whiskers graph: line = median, box = interquartile range (25%–75%), and the whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box. (d) Immunoblotting analysis of striatins and pMST3&4 on siRNA depletion in MDA-MB-231 cells. (e) Tumour Cell Genome Atlas (TCGA) data on cBioportal demonstrating the frequency of mutation, deletion, amplification, and multiple alteration for human FAM40A/STRIP1 and FAM40B/STRIP2.
Supplementary Figure 7 Spatial ERM translocation is the outcome but not the course of the morphological changes observed on FAM40A&B, MST3&4 and CCM3 depletion.
Stable ectopic overexpression of wild type Ezrin-GFP or constitutive active Ezrin-T567D-GFP (EzrinTD) in A431 cells did not rescue the cell morphology changes observed on FAM40A, FAM40B, MST3&4, and CCM3 depletion. All experiments were conducted at least 3 independent times. Scale bar, 10 μm.
Supplementary Figure 8 PPP1CB and PPP1R14C regulate cell morphology.
(a) Characterisation of morphological phenotype in MDA-MB-231 cells on siRNA depletion of five phosphatases used in the original screen. F-actin is stained with Phalloidin-TRITC (red) and the nucleus is stained with DAPI. (b) The phospho-specific PPP1R14A antibody is a pan-PPP1R14 antibody as it also binds specifically to overexpressed wild type PPP1R14C but not the T73A point mutant. (c) Serum stimulation of serum starved A431 cells stimulates translocation and phosphorylation of PPP1R14 at cell-cell junction and cell edges with the same kinetics as MST3 translocation. (d) Immunofluorescence analysis of pS19-MLC (green), F-actin (red) and β-catenin (blue) in PPP1R14C depleted A431 cells. (e) Quantification of A431 cell area +/−s.e.m on depletion of PPP1R14C using siRNA smart pools (n = fields of cells; Ctr, 31; 14C, 27). Statistical test were performed using unpaired student’s t-test (two-tailed), ∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001. All experiments were conducted at least 3 independent times. Scale bars, 10 μm. (f) Working model. The STRIPAK complex controls the spatial regulation of actomyosin and ERM activities at cell-cell junction and cell edges. The STRIPAK complex thereby acts in synergy with other regulators of contractions, such as RhoA/ROCK. The STRIPAK complex is composed of PP2A phosphatases that can inactivate the kinases MST3&4 within the complex. PP2A is directed to the activation loop of the kinases by the scaffolding proteins FAM40A and FAM40B1, while FAM40B2 cannot bind PP2A catalytic sub-units and acts as a competitive inhibitor. CCM3 recruits MST3&4 to sites of actomyosin contraction where it phosphorylates and activates members the PP1 phosphatase inhibitor family, PPP1R14A-D, leading to increased contraction. MST3&4 may also directly phosphorylate ERM proteins. Ultimately, the STRIPAK complex determines if the actomyosin network is coupled to the plasma membrane (ERM). Low MST3&4 activities couple the actomyosin network to the ECM and favour 2D migration, while high MST3&4 activities couple the actomyosin network to the plasma membrane facilitating 3D migration through confined environment.
Supplementary information
Supplementary Information
Supplementary Information (PDF 9929 kb)
Supplementary Table 1: Information of the siRNA screen's.
Sheet 1. Fly screen. List of genes that were depleted in the fly screen. Sheet 2. Fly genes and human homologous. Genes with similarities are also included. Sheet 3. siRNA sequences and catalogue number of all siRNA used in the screen. All siRNA's were purchased by Dharmacon. Sheet 4. Fly and human gene names, fly and A431 phenotypes. (XLSX 59 kb)
Supplementary Table 2: Peptide kinase screen.
Peptide sequences and specific phospho site are shown. Raw data from each experiment is shown. (XLSX 13 kb)
Supplementary Table 3: qPCR primers and siRNA oligo's.
qPCR primer sequences and siRNAs used in the study including catalog numbers. (XLSX 47 kb)
Supplementary Table 4: Expression vectors.
All expression vectors used in this study are shown. (XLSX 10 kb)
Supplementary Table 5: Antibodies.
All antibodies used in this study including provider, catalog numbers and dilutions are shown. (XLSX 11 kb)
3D morphologies of siRNA depleted A431 cells.
3D reconstruction of confocal stacks taken of siRNA transfected A431 cells stained for F-actin (red) and pS19-MLC (green). The cells were plated on top of collagen-1/matrigels. The movie includes siCtr, siFAM40A, siFAM40B and STRN3 depleted A431 cells sequentially. (AVI 5809 kb)
Spatiotemporal regulation of MST3-GFP.
Confocal time lapse movie of siRNA transfected A431-MST3-GFP cells. The cells have been serum starved for 24 h and then stimulated with FBS. Imaging is then initiated immediately and frames are taken every 20 s. When cells were treated with ROCK inhibitor (Y27632) the drug was added during serum starvation. The movie includes siCtr, ROCK inhibitor (Y27632) treated, and siCCM3 depleted A431-MST3-GFP cells sequentially. (AVI 2386 kb)
Time-lapse movie of siRNA depleted MDA-MD231 cells on hard surfaces.
Phase contrast time lapse movie of siRNA transfected MDA-MB-231 cells plated on a 2D planar surface. Images were taken every 5 min. The movie includes siCtr, siFAM40A, siFAM40B and siMST3&4 depleted MDA-MB-231 cells sequentially. (AVI 15402 kb)
Time-lapse movie of siRNA depleted MDA-MD231 cells on soft surfaces.
Phase contrast time lapse movie of siRNA transfected MDA-MB-231 cells plated on top of collagen-I/matrigels. Images were taken every 5 min. The movie includes siCtr, siFAM40A, siFAM40B and siMST3&4 depleted MDA-MB-231 cells sequentially. (AVI 16369 kb)
Rights and permissions
About this article
Cite this article
Madsen, C., Hooper, S., Tozluoglu, M. et al. STRIPAK components determine mode of cancer cell migration and metastasis. Nat Cell Biol 17, 68–80 (2015). https://doi.org/10.1038/ncb3083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3083
This article is cited by
-
STRIP2 motivates non-small cell lung cancer progression by modulating the TMBIM6 stability through IGF2BP3 dependent
Journal of Experimental & Clinical Cancer Research (2023)
-
Molecular mechanisms involved in regulating protein activity and biological function of MST3
Cell Division (2023)
-
Notch-based gene signature for predicting the response to neoadjuvant chemotherapy in triple-negative breast cancer
Journal of Translational Medicine (2023)
-
Osimertinib resistance prognostic gene signature: STRIP2 is associated with immune infiltration and tumor progression in lung adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
MST4 negatively regulates type I interferons production via targeting MAVS-mediated pathway
Cell Communication and Signaling (2022)