Abstract
Cyclin C was cloned as a growth-promoting G1 cyclin, and was also shown to regulate gene transcription. Here we report that in vivo cyclin C acts as a haploinsufficient tumour suppressor, by controlling Notch1 oncogene levels. Cyclin C activates an ‘orphan’ CDK19 kinase, as well as CDK8 and CDK3. These cyclin-C–CDK complexes phosphorylate the Notch1 intracellular domain (ICN1) and promote ICN1 degradation. Genetic ablation of cyclin C blocks ICN1 phosphorylation in vivo, thereby elevating ICN1 levels in cyclin-C-knockout mice. Cyclin C ablation or heterozygosity collaborates with other oncogenic lesions and accelerates development of T-cell acute lymphoblastic leukaemia (T-ALL). Furthermore, the cyclin C encoding gene CCNC is heterozygously deleted in a significant fraction of human T-ALLs, and these tumours express reduced cyclin C levels. We also describe point mutations in human T-ALL that render cyclin-C–CDK unable to phosphorylate ICN1. Hence, tumour cells may develop different strategies to evade inhibition by cyclin C.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Leopold, P. & O’Farrell, P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell 66, 1207–1216 (1991).
Lew, D. J., Dulic, V. & Reed, S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66, 1197–1206 (1991).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Liu, Z. J. et al. A critical role for cyclin C in promotion of the hematopoietic cell cycle by cooperation with c-Myc. Mol. Cell Biol. 18, 3445–3454 (1998).
Makkonen, K. M., Malinen, M., Ropponen, A., Vaisanen, S. & Carlberg, C. Cell cycle regulatory effects of retinoic acid and forskolin are mediated by the cyclin C gene. J. Mol. Biol. 393, 261–271 (2009).
Miyata, Y. et al. Cyclin C regulates human hematopoietic stem/progenitor cell quiescence. Stem Cells 28, 308–317 (2010).
Ren, S. & Rollins, B. J. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117, 239–251 (2004).
Saxena, U. H. et al. Phosphorylation by cyclin C/cyclin-dependent kinase 2 following mitogenic stimulation of murine fibroblasts inhibits transcriptional activity of LSF during G1 progression. Mol. Cell Biol. 29, 2335–2345 (2009).
Hengartner, C. J. et al. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol. Cell 2, 43–53 (1998).
Leclerc, V., Tassan, J. P., O’Farrell, P. H., Nigg, E. A. & Leopold, P. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol. Biol. Cell 7, 505–513 (1996).
Liao, S. M. et al. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374, 193–196 (1995).
Maldonado, E. et al. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature 381, 86–89 (1996).
Rickert, P., Seghezzi, W., Shanahan, F., Cho, H. & Lees, E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene 12, 2631–2640 (1996).
Tassan, J. P., Jaquenoud, M., Leopold, P., Schultz, S. J. & Nigg, E. A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl Acad. Sci. USA 92, 8871–8875 (1995).
Akoulitchev, S., Chuikov, S. & Reinberg, D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407, 102–106 (2000).
Elmlund, H. et al. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc. Natl Acad. Sci. USA 103, 15788–15793 (2006).
Knuesel, M. T., Meyer, K. D., Bernecky, C. & Taatjes, D. J. The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function. Genes Dev. 23, 439–451 (2009).
Alarcon, C. et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139, 757–769 (2009).
Chi, Y. et al. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15, 1078–1092 (2001).
Fryer, C. J., White, J. B. & Jones, K. A. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol. Cell 16, 509–520 (2004).
Nelson, C., Goto, S., Lund, K., Hung, W. & Sadowski, I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421, 187–190 (2003).
Donner, A. J., Szostek, S., Hoover, J. M. & Espinosa, J. M. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol. Cell 27, 121–133 (2007).
Firestein, R. et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 455, 547–551 (2008).
Morris, E. J. et al. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8. Nature 455, 552–556 (2008).
Donner, A. J., Ebmeier, C. C., Taatjes, D. J. & Espinosa, J. M. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat. Struct. Mol. Biol. 17, 194–201 (2010).
Furumoto, T. et al. A kinase subunit of the human mediator complex, CDK8, positively regulates transcriptional activation. Genes Cells 12, 119–132 (2007).
Li, H. et al. Molecular cloning and chromosomal localization of the human cyclin C (CCNC) and cyclin E (CCNE) genes: deletion of the CCNC gene in human tumors. Genomics 32, 253–259 (1996).
Ohata, N. et al. Highly frequent allelic loss of chromosome 6q16-23 in osteosarcoma: involvement of cyclin C in osteosarcoma. Int. J. Mol. Med. 18, 1153–1158 (2006).
Bondi, J. et al. Expression and gene amplification of primary (A, B1, D1, D3, and E) and secondary (C and H) cyclins in colon adenocarcinomas and correlation with patient outcome. J. Clin. Pathol. 58, 509–514 (2005).
Galamb, O. et al. Evaluation of malignant and benign gastric biopsy specimens by mRNA expression profile and multivariate statistical methods. Cytometry B Clin. Cytom. 72, 299–309 (2007).
Husdal, A., Bukholm, G. & Bukholm, I. R. The prognostic value and overexpression of cyclin A is correlated with gene amplification of both cyclin A and cyclin E in breast cancer patient. Cell Oncol. 28, 107–116 (2006).
Iqbal, J. et al. Clinical implication of genome-wide profiling in diffuse large B-cell lymphoma and other subtypes of B-cell lymphoma. Indian J. Cancer 44, 72–86 (2007).
Xu, W. & Ji, J. Y. Dysregulation of CDK8 and Cyclin C in tumorigenesis. J. Genet. Genomics 38, 439–452 (2011).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Ye, X., Zhu, C. & Harper, J. W. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl Acad. Sci. USA 98, 1682–1686 (2001).
Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Malumbres, M. Physiological relevance of cell cycle kinases. Physiol. Rev. 91, 973–1007 (2011).
Welcker, M. & Clurman, B. E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8, 83–93 (2008).
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Thompson, B. J. et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J. Exp. Med. 204, 1825–1835 (2007).
Chiang, M. Y. et al. Leukemia-associated NOTCH1 alleles are weak tumor initiators but accelerate K-ras-initiated leukemia. J. Clin. Invest. 118, 3181–3194 (2008).
Boehm, T., Foroni, L., Kaneko, Y., Perutz, M. F. & Rabbitts, T. H. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc. Natl Acad. Sci. USA 88, 4367–4371 (1991).
Larson, R. C. et al. T cell tumours of disparate phenotype in mice transgenic for Rbtn-2. Oncogene 9, 3675–3681 (1994).
McGuire, E. A., Rintoul, C. E., Sclar, G. M. & Korsmeyer, S. J. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol. Cell Biol. 12, 4186–4196 (1992).
Lin, Y. W., Nichols, R. A., Letterio, J. J. & Aplan, P. D. Notch1 mutations are important for leukemic transformation in murine models of precursor-T leukemia/lymphoma. Blood 107, 2540–2543 (2006).
O’Neil, J. et al. Activating Notch1 mutations in mouse models of T-ALL. Blood 107, 781–785 (2006).
Hyytinen, E. R. et al. Defining the region(s) of deletion at 6q16-q22 in human prostate cancer. Genes Chromosomes Cancer 34, 306–312 (2002).
Orphanos, V. et al. Allelic imbalance of chromosome 6q in ovarian tumours. Br. J. Cancer 71, 666–669 (1995).
Utada, Y. et al. Mapping of target regions of allelic loss in primary breast cancers to 1-cM intervals on genomic contigs at 6q21 and 6q25.3. Jpn. J. Cancer Res. 91, 293–300 (2000).
Zhang, Y. et al. A 3-cM commonly deleted region in 6q21 in leukemias and lymphomas delineated by fluorescence in situ hybridization. Genes Chromosomes Cancer 27, 52–58 (2000).
Mansour, M. R. et al. Prognostic implications of NOTCH1 and FBXW7 mutations in adults with T-cell acute lymphoblastic leukemia treated on the MRC UKALLXII/ECOG E2993 protocol. J. Clin. Oncol. 27, 4352–4356 (2009).
Koch, U. & Radtke, F. Notch signaling in solid tumors. Curr. Top Dev. Biol. 92, 411–55 (2010).
Jackson, A. et al. Deletion of 6q16-q21 in human lymphoid malignancies: a mapping and deletion analysis. Cancer Res. 60, 2775–2779 (2000).
Sinclair, P. B. et al. A fluorescence in situ hybridization map of 6q deletions in acute lymphocytic leukemia: identification and analysis of a candidate tumor suppressor gene. Cancer Res. 64, 4089–4098 (2004).
Lobry, C., Oh, P., Mansour, M. R., Look, A. T. & Aifantis, I. Notch signaling: switching an oncogene to a tumor suppressor. Blood 123, 2451–2459 (2014).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33, 416–421 (2003).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
King, B. et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell 153, 1552–1566 (2013).
Li, X., Gounari, F., Protopopov, A., Khazaie, K. & von Boehmer, H. Oncogenesis of T-ALL and nonmalignant consequences of overexpressing intracellular NOTCH1. J. Exp. Med. 205, 2851–2861 (2008).
Chiang, M. Y. et al. Identification of a conserved negative regulatory sequence that influences the leukemogenic activity of NOTCH1. Mol. Cell Biol. 26, 6261–6271 (2006).
Inuzuka, H. et al. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCFβTRCP ubiquitin ligase. Cancer Cell 18, 147–159 (2010).
Emmerich, C. H. et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc. Natl Acad. Sci. USA 110, 15247–15252 (2013).
Vrooman, L. M. et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J. Clin. Oncol. 31, 1202–1210 (2013).
Winter, S. S. et al. Identification of genomic classifiers that distinguish induction failure in T-lineage acute lymphoblastic leukemia: a report from the children’s oncology group. Blood 110, 1429–1438 (2007).
Gutierrez, A. et al. Inactivation of LEF1 in T-cell acute lymphoblastic leukemia. Blood 115, 2845–2851 (2010).
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Mansour, M. R. et al. Notch-1 mutations are secondary events in some patients with T-cell acute lymphoblastic leukemia. Clin. Cancer Res. 13, 6964–6969 (2007).
Mansour, M. R., Linch, D. C., Foroni, L., Goldstone, A. H. & Gale, R. E. High incidence of Notch-1 mutations in adult patients with T-cell acute lymphoblastic leukemia. Leukemia 20, 537–539 (2006).
Acknowledgements
We thank S. Blacklow, T. Sanda, T. Roberts, E. Sicinska, I. Kalaszczynska, S. Bukarac and D. Payne-Turner for help at different stages of the project. This work was supported by grants P01 CA109901 (to P.S., H.v.B. and A.T.L.), R01 CA083688 (to P.S.), GM094777 (to W.W.), American Syrian Lebanese Associated Charities of St. Jude Children’s Research Hospital (to C.G.M.), and R01 AG011085 (to J.W.H.). C.G.M. is a St. Baldrick’s Scholar and Pew Scholar.
Author information
Authors and Affiliations
Contributions
N.L. and P.S. conceived the study, analysed and interpreted the data and wrote the paper. N.L. performed experiments described in the study with fundamental help from: A.F. who contributed phenotypic, in vivo molecular and tumour analyses, and contributed to writing the manuscript, J.C. and S.P.G. contributed all mass spectrometry analyses, H.I., S.S. and W.W. contributed molecular mechanistic analyses of the cyclin C → Fbw7 → ICN1 link, X.L. and H.v.B. contributed bone marrow transduction with ICN1 experiments, T.K. and H.v.B. performed analyses of thymocyte populations and studies of bone marrow chimaeras. M.R.M., S.J., R.E.G. and D.C.L. discovered mutations in human patients that render cyclin C–CDK unable to phosphorylate Notch1, L.L. contributed to molecular studies and phosphorylation analyses, H.W. performed all analyses of ICN1 phosphorylation by cyclin C–CDK8, C–CDK19 and C–CDK3, B.K. and I.A. contributed in vivo mouse tumorigenesis studies, A.G. and A.T.L. contributed analyses of human T-ALL samples, A.O. and J.W.H. performed analyses of endogenous ICN1 polyubiquitylation, T.O. contributed shRNA analyses of mouse cells and analyses of pRB phosphorylation, L. Baitsch and J.J.Z. helped with molecular in vivo analyses, C.A.M. analysed gene expression data, M.J.K. and J.C.A. performed immunostaining for ICN1, M.R. and J.C.A. contributed analyses of anti-phospho ICN1 antibody, S.K. helped with analyses of human T-ALL, X.Z. and J.C.A. developed the anti-phospho-ICN1 antibody, C.G.M. contributed DNA sequencing and copy number analyses of the cyclin C gene in human T-ALL, L. Bury, N.K., K.A.M., Y.G. and A.Z. helped N.L. at different stages of the project. A.F., J.C., H.I., X.L. and M.R.M. contributed equally. P.S. directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Analyses of hematopoietic compartments in cyclin CD/D mice. Ctrl and C-KO denote pI-pC treated cyclin CF/F/Mx1-Cre− and CΔ/Δ/Mx1-Cre+ mice, respectively.
(a) Western blot analysis of cyclin C in the indicated organs. BM, bone marrow. (b) FACS analysis of thymocytes. Cells were stained with anti-CD4 and-CD8 antibodies. (c,d) Mean proportion of the indicated thymocyte subsets (n = 4 per genotype). DN, double-negative (please see Methods for staining). CD4 + /CD8 + P = 0.108, CD4 + P = 0.093, CD8 + P = 0.201, CD4-/CD8- P = 0.0004, DN1 P = 0.225, DN2 P = 0.253, DN3 P = 0.0007, DN3 to DN4 P = 0.0013, DN4 P = 0.052 (t-test). (e) Protein lysates prepared from thymocytes were analyzed by western blotting. Each lane corresponds to a separate animal of the indicated genotype. Note increased ICN1 levels in C-KO thymocytes. The levels of other regulators of thymocyte proliferation (cyclin D3, CDK6) were unchanged, or only slightly increased in C-KO. (f) Thymocytes were isolated from control and cyclin C-KO mice and analyzed by western blotting. Note that the levels of F-box protein Fbw7 (which controls ICN1 degradation) were not changed in C-KO cells. Moreover, the levels of other Fbw7 targets (Mcl1, cyclin E1, c-Myc) were not changed in C-KO cells. Also knockdown of cyclin C in human T-ALL cells had essentially no effect on the levels or half-life of these proteins (Supplementary Fig. 3c). Hence ablation of cyclin C selectively affects the stability of ICN1, but not of other Fbw7 substrates. (g) Numbers of red blood cells, white blood cells and platelets in peripheral blood (n = 6 per group). Each dot corresponds to a separate animal, horizontal lines represent mean values. Red blood cells P = 0.36, white blood cells P = 0.40, platelets P = 0.001 (t-test). (h) Mean total number of nucleated bone marrow cells (per one femur and tibia, n = 5 per group). Error bars, S.D. P = 0.21 (t-test). (i) Mean percentages of long-term hematopoietic stem cells (LT-HSC), short-term hematopoietic stem cells (ST-HSC), lymphoid-primed multipotent progenitors (LMPP) and common lymphoid progenitors (CLP) in bone marrow (n = 4 per group). Error bars, S.D. LT-HSC P = 0.958, ST-HSC P = 0.603, LMPP P = 0.993, CLP P = 0.488 (t-test). (j) Lin− bone marrow cells were cultured for 8 days on OP9-DL1 stroma. Cells were then stained for CD11b, CD19, CD4, CD8, CD25 and CD44, and analyzed by FACS. Shown are mean percentages of in vitro cultured cells displaying the indicated immunophenotype. Lin− bone marrow was pooled from 4 mice per group. Data represent 2 technical replicates.
Supplementary Figure 2 Analyses of ICN1 stability in cyclin CΔ/Δ thymocytes.
(a) Thymocytes were isolated from control (Ctrl, cyclin CF/F/Mx1-Cre−) and cyclin C-KO (cyclin CΔ/Δ/Mx1-Cre) animals and treated with protein synthesis inhibitor cycloheximide (CHX, 100 μg/ml) for the indicated times. The levels of ICN1 were then examined by western blotting. (b) Thymocytes isolated from control and cyclin C-KO animals (genotypes as in a) were treated with a γ-secretase inhibitor Compound E (200 nM) for the indicated times. The levels of ICN1 were then examined by western blotting. (c) Thymocytes isolated from control and cyclin C-KO animals (genotypes as in a) were treated with proteasome inhibitors MG132 (10 μm), MG132 + lactacystin (MG132 + Lac, 10 μm), or vehicle only (DMSO) for 6 h. Cell lysates were then prepared, and analyzed for ICN1 levels by western blotting.
Supplementary Figure 3 Analyses of human T-cell acute lymphoblastic leukemia (T-ALL) cells.
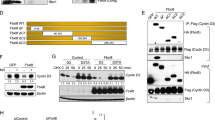
(a) Human T-ALL MOLT-16 cells were transduced with viruses expressing anti-cyclin C shRNA or control shRNA (CTR). Shown are mean percentages of cells in the indicated cell cycle phases, error bars denote standard deviation of triplicate samples. G1 CTR to G1 cyclin C shRNA P = 0.052, S CTR to S cyclin C shRNA P = 0.993, G2/M CTR to G2/M cyclin C shRNA P = 0.151 (t-test). (b) MOLT-16 cells were transduced with anti-cyclin C or control shRNAs (CTR). Cells were harvested and lysates probed with the indicated antibodies. (c) MOLT-16 cells were transduced with viruses expressing anti-cyclin C shRNAs (1, 2 or 3) or control shRNA (CTR). Cycloheximide (20 μg/ml) was added to the media and cells were harvested at the indicated time-points. Lysates were immunoblotted with the indicated antibodies. Note that knockdown of cyclin C strongly increased the half-life of ICN1, but it had no major effect on half-lives of other Fbw7 targets (c-Myc, cyclin E, mTOR, Mcl1; see also Supplementary Fig. 1f). The half-life of ICN1 in cells depleted of cyclin C and in control cells was determined by densitometric analysis (ImageJ, NIH). (d) Combined knock-down of cyclin C and Fbw7. MOLT-16 cells were transduced with lentiviral vectors expressing shRNA against Fbw7 or cyclin C, or both. Lysates were immunoblotted using the indicated antibodies. Note that knockdown of cyclin C led to upregulation of ICN1 (compare ICN1, lanes 1 versus 2). Knockdown of Fbw7 also increased ICN1 levels, as expected (compare ICN1, lanes 1 versus 3). Importantly, cyclin C-knockdown did not further increase ICN1 levels in cells depleted of Fbw7 (ICN1, lanes 3 versus 4), indicating that the two proteins operate in the same pathway. Fbw7-knockdown increased the levels of an Fbw7 target, cyclin E (compare lanes 1 versus 3). (e) MOLT-16 cells were transduced with viruses encoding cyclin C, CDK8, or with empty vectors (EV). The levels of the indicated proteins were determined by immunoblotting. (f) MOLT-16 cells were transduced with viruses encoding HA-tagged cyclin C, CDK8, CDK3, CDK19 or empty vectors (EV). The levels of the indicated proteins were determined by immunoblotting. For detection of HA-tagged proteins, an anti-HA antibody was used. This antibody detects a background band that co-migrates with HA-CDK19 (star).
Supplementary Figure 4 Cyclin C-CDK8, C-CDK19 and C-CDK3 kinases regulate ICN1.
(a) Protein sequences for human CDK8 (GI: 4502745), CDK19 (GI: 30387611), and CDK3 (GI: 4557439) were obtained from NCBI RefSeq. Multiple sequence alignment was performed with MAFFT (version 7) using the L-INS-i method. The resulting alignment was visualized using Jalview 2.8, highlighting identical amino acids between all three kinases in dark blue, and identical amino acids between two of the three kinases in light blue. (b–d) MOLT-16 cells were transduced with viruses encoding shRNAs against the indicated CDKs. The levels of ICN1 and CDKs were then determined by immunoblotting. (e) Validation of anti-CDK3 shRNAs. HeLa cells stably expressing HA-tagged CDK3 were used (to allow unequivocal detection of CDK3 with anti-HA antibody). Cells were transduced with viruses expressing five different anti-CDK3 shRNAs (1-5). Knockdown of CDK3 was gauged by immunoblotting with an anti-HA antibody. shRNAs #2 and #3 were chosen and used for analyses shown in d (f) His-tagged CDK19, or cyclin C, or cyclin C plus His-tagged CDK19, were expressed in 293T cells. Complexes were immunoprecipitated using anti-His antibody (or with IgG, for control) and used in in vitro kinase reactions in the presence (+) or absence (−) of ICN1 as a substrate together with γ[32P]ATP. As a positive control, ICN1 was incubated with recombinant cyclin-C-CDK8. Upper panel: proteins analyzed by autoradiography to detect phosphorylated ICN1 (32P-ICN1). Second panel: total GST-ICN1 protein detected by immunoblotting with anti-GST antibody (ICN1). Third and fourth panels: CDK19 and cyclin C detected using the indicated antibodies (anti-His antibody was used to detect tagged CDK19). (g) Whole cell lysates (Input) from experiment shown in f, immunoblotted with the indicated antibodies. Anti-His antibody was used to detect tagged CDK19. (h) HA-tagged CDK3, or cyclin C, or cyclin C plus HA-tagged CDK3 were expressed in 293T cells. Complexes were immunoprecipitated using anti-HA antibody (or with IgG, for control), and used in in vitro kinase reactions in the presence (+) or absence (−) of ICN1 as a substrate together with γ[32P]ATP. As a positive control, ICN1 was incubated with recombinant cyclin C-CDK8. Upper panel: proteins analyzed by autoradiography to detect phosphorylated ICN1 (32P-ICN1). Second panel: total GST-ICN1 proteins detected by Ponceau S staining. Third and fourth panels: CDK3 and cyclin C were detected using the indicated antibodies (anti-HA antibody was used to detect tagged CDK3). (i) Whole cell lysates (Input) from experiment shown in h, immunoblotted with the indicated antibodies. Anti-HA antibody was used to detect tagged CDK3.
Supplementary Figure 5 Cyclin C-CDK19 and C-CDK3 kinases phosphorylate T2512, S2514 and S2517 of ICN1.
Cyclin C-CDK19 or cyclin C-CDK3 complexes purified from Sf9 cells were incubated with N-EHPFLTPSPESPDQWFPK-C peptide, corresponding to aminoacids 2507 to 2521 of Notch1, as described in Methods. Separation of these Notch1 peptides by liquid chromatography after in vitro kinase reactions demonstrated three distinct peaks, each representing one of the three in vivo identified Notch1 phosphorylation sites (T2512, S2514 and S2517). Each of these identified phosphorylation sites could be confirmed by MS/MS analysis and sites localized using an Ascore localization score. Representative MS/MS scans are shown for both cyclin C-CDK19 and cyclin C-CDK3 experiments.
Supplementary Figure 6 Molecular analyses of cyclin C function.
(a,b) Characterization of anti-phospho-Ser2517 ICN1 antibody. (a) Myc-taggedwild-type ICN1 (WT), or the indicated ICN1 mutants (TSS denotes T2512/S2514A/S2517A triple-mutant), or empty vectors (EV) were transfected into 293T cells. Cell lysates were probed with an anti-phospho-ICN1 antibody. (b) Upper panel: wild-type ICN1 (WT), or the indicated ICN1 mutants were expressed in 293T cells, immunoprecipitated with anti-Notch1 antibody, and immunoblotted with anti-phospho-ICN1 antibody. Lower panel: Notch1 was immunoprecipitated from I22 cells [a murine T-ALL line derived from a tumor induced by retrovirally-driven ICN1 (Pear et al. J. Exp. Med. 1996;183:2283)]. Immunoprecipitates were treated with lambda phosphatase (+) or left untreated (−), immunoblotted and probed with an anti-phosho-ICN1 antibody. Phosphatase treatment abolished immunoreactivity, as expected. (c) MOLT-16 cells were transduced with anti-cyclin C or control shRNA (CTR). ICN1 was immunoprecipitated (IP); normal IgG IP was used as a negative control. Immunoblots were probed with the indicated antibodies. Note reduced phosphorylation of endogenous ICN1, and reduced interaction of endogenous ICN1 and Fbw7 proteins in cyclin C-depleted cells. Input, immunoblotting of straight lysates. (d) Cyclin CF/F MEFs were transduced with Myc-tagged-ICN1. Cells were then treated with control adenovirus (CF/F) or with an adenovirus encoding Cre (CΔ/Δ). ICN1-Myc was immunoprecipitated and immunoblots were probed using an antibody against Fbw7, or against Myc. Input, whole cell lysates. Note that the interaction between ICN1 and endogenous Fbw7 was strongly decreased on ablation of cyclin C. (e) HeLa cells were transfected with HA-tagged Fbw7 along with an empty vector (EV), or Myc-tagged wild-type ICN1 (WT), or various ICN mutants containing alanine-substitutions within critical cyclin C-CDK-dependent phosphosites(TSS denotes T2512/S2514A/S2517A triple-mutant). ICN1 was immunoprecipitated using an anti-Myc antibody, and immunoblots were probed with an anti-HA antibody (to detect ICN1-bound Fbw7). Input, whole cell extracts. Note that alanine-substitutions inhibited the interaction of ICN1 with Fbw7 in vivo. (f) Cyclin CF/F MEF were transduced with Notch1 (WT-Notch1) or a Notch1 mutant (TSS-Notch1) in which the three critical cyclin C-CDK phosphorylation sites have been mutated to alanines (T2512A, S2514A S2517A). Cells were then transduced with control adenovirus (CF/F), or an adenovirus encoding Cre (CΔ/Δ). The levels of ICN1 were determined by western blotting. The middle portion of the gel was cut out and the images spliced together (dashed line). The expression of alanine-substituted mutant was strongly, as compared to the wild-type protein, and it was no longer affected by cyclin C-ablation. (g) HEK293 cells were transduced with anti-cyclin C or control shRNA (CTR). Cells were then transfected with Myc-tagged-ICN1 and His-tagged-ubiquitin. Ubiquitinated proteins were immunoprecipitated using Ni-NTA matrices and immunoblots were probed with an anti-Myc antibody to detect ubiquitinated ICN1. Input, whole cell lysates. Densitometric scanning of ubiquitinated ICN1 band intensities (normalized against input Myc-ICN1) revealed approximately 3-fold lower ubiquitination of ICN1 in cells depleted of cyclin C.
Supplementary Figure 7 Analyses of cyclin C function in T-ALL.
(a) HPC were isolated from pI-pC-treated CF/F/Mx1-Cre− (Ctrl), C+/Δ/Mx1-Cre (C-HET) and cyclin CΔ/Δ/Mx1-Cre (C-KO) animals. pRB phosphorylation at Ser807/811 [a residue phosphorylated by cyclin C-CDK3 and D-CDK4 in human cells (Ren and Rollins, Cell 2004; 117:239; Zarkowska and Mittnacht, J. Biol. Chem. 1997; 272:12738) was analyzed by immunoblotting. (b) HPC were isolated from mice of the indicated genotypes (as in a) and left untreated (Lin-BM), or cultured for 3 days on OP9-DL1 stroma (Lin-BM/OP9-DL1). Alternatively, HPC were transduced with Notch1-P12 (Lin-BM/Notch1-P12) or ICN1 (Lin-BM/ICN1). Cells were pulsed with BrdU and analyzed by FACS. Shown is the mean percentage of BrdU-positive cells; error bars, S.D. HPC (Lin−) were pooled from 4 mice per group. Data represent 2 biological replicates. (c) Hematoxylin and eosin stained sections of organs collected at 4.5 wks post bone marrow transplantation, from mice which received ICN1-transduced control and C-KO HPC. Note the presence of infiltrating tumor cells (arrows) in recipients of C-KO cells, but not control cells. Scale bar, 500 μm. (d) Organs were collected from recipients of ICN1-transduced Ctrl and C-KO HPC. Animals were injected with BrdU, GFP+ tumor cells were sorted, and the percentage of BrdU-positive cells was evaluated by FACS. Each dot corresponds to a separate animal. Horizontal lines denote mean values, error bars, SD. P = 0.2452 for bone marrow, P = 0.0114 for spleen, P = 0.1554 for thymus (unpaired t-test). (e) HPC from Ctrl and C-KO mice (genotypes as in a) were transduced with Notch1-P12 and GFP, and injected into recipient mice. The percentage of leukemic cells (GFP+, CD4/CD8+) in peripheral blood was evaluated by FACS after 7 weeks. Each dot corresponds to a separate animal, horizontal lines denote mean values. p = 0.0171 (Mann–Whitney test). (f) Similar experiment as in Fig. 6h in the main text, except that control and cyclin C-KO HPC were transduced with a mutant version of Notch1 (ΔEGFΔLNR). This mutant lacks C-terminal aminoacids 2473-2556, which harbor the three essential cyclin C-CDK phosphorylation sites. Left: tumor incidence, n = 10 per group. Right: Kaplan–Meier survival analysis, n = 10 per group. (g) CDK19 expression was analyzed using gene expression microarrays on a subset of primary T-ALL patient samples shown in the main text in Fig. 7a.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2123 kb)
Supplementary Table 1
Supplementary Information (XLSX 58 kb)
Supplementary Table 2
Supplementary Information (XLSX 28 kb)
Supplementary Table 3
Supplementary Information (XLSX 514 kb)
Rights and permissions
About this article
Cite this article
Li, N., Fassl, A., Chick, J. et al. Cyclin C is a haploinsufficient tumour suppressor. Nat Cell Biol 16, 1080–1091 (2014). https://doi.org/10.1038/ncb3046
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3046
This article is cited by
-
FBP1 inhibits NSCLC stemness by promoting ubiquitination of Notch1 intracellular domain and accelerating degradation
Cellular and Molecular Life Sciences (2024)
-
Disruption to the FOXO-PRDM1 axis resulting from deletions of chromosome 6 in acute lymphoblastic leukaemia
Leukemia (2023)
-
In vivo CRISPR screens reveal Serpinb9 and Adam2 as regulators of immune therapy response in lung cancer
Nature Communications (2023)
-
MED12 is overexpressed in glioblastoma patients and serves as an oncogene by targeting the VDR/BCL6/p53 axis
Cellular and Molecular Life Sciences (2022)
-
Construction of a prognostic model with histone modification-related genes and identification of potential drugs in pancreatic cancer
Cancer Cell International (2021)