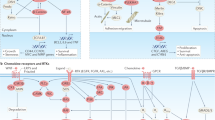
Abstract
Recent pre-clinical and clinical research has provided evidence that cancer progression is driven not only by a tumour's underlying genetic alterations and paracrine interactions within the tumour microenvironment, but also by complex systemic processes. We review these emerging paradigms of cancer pathophysiology and discuss how a clearer understanding of systemic regulation of cancer progression could guide development of new therapeutic modalities and efforts to prevent disease relapse following initial diagnosis and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Pietras, K. & Ostman, A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 316, 1324–1331 (2010).
Allinen, M. et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6, 17–32 (2004).
Chang, H. Y. et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2, E7 (2004).
Finak, G. et al. Gene expression signatures of morphologically normal breast tissue identify basal-like tumors. Breast Cancer Res. 8, R58 (2006).
Casey, T. et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res. Treat. 114, 47–62 (2009).
Ma, X. J., Dahiya, S., Richardson, E., Erlander, M. & Sgroi, D. C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 11, R7 (2009).
Zajicek, G. Cancer as a systemic disease. Med. Hypotheses 4, 193–207 (1978).
Redig, A. J. & McAllister, S. S. Breast cancer as a systemic disease: a view of metastasis. J. Intern. Med. 274, 113–126 (2013).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Arwert, E. N., Hoste, E. & Watt, F. M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 12, 170–180 (2012).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
De Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 6, 24–37 (2006).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Trinchieri, G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 30, 677–706 (2012).
Kalluri, R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 3, 422–433 (2003).
Lech, M. & Anders, H. J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 1832, 989–997 (2013).
Polyak, K., Haviv, I. & Campbell, I. G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 25, 30–38 (2009).
Marsh, T., Pietras, K. & McAllister, S. S. Fibroblasts as architects of cancer pathogenesis. Biochim. Biophys. Acta 1832, 1070–1078 (2012).
Bissell, M. J. & Radisky, D. Putting tumours in context. Nat. Rev. Cancer 1, 46–54 (2001).
Seruga, B., Zhang, H., Bernstein, L. J. & Tannock, I. F. Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer 8, 887–899 (2008).
Gao, D. & Mittal, V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol. Med. 15, 333–343 (2009).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Cuiffo, B. G. & Karnoub, A. E. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh. Migr. 6, 220–230 (2012).
Shaked, Y., McAllister, S., Fainaru, O. & Almog, N. Tumor dormancy and the angiogenic switch: possible implications of bone marrow-derived cells. Curr. Pharm. Des. 19, 1–14 (2014).
Eash, K. J., Greenbaum, A. M., Gopalan, P. K. & Link, D. C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 (2010).
Martin, C. et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 (2003).
Wright, D. E., Wagers, A. J., Gulati, A. P., Johnson, F. L. & Weissman, I. L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003).
Christopher, M. J., Rao, M., Liu, F., Woloszynek, J. R. & Link, D. C. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 208, 251–260 (2011).
Chow, A. et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208, 261–271 (2011).
Winkler, I. G. et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 (2010).
Shojaei, F. et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25, 911–920 (2007).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
McAllister, S. S. et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133, 994–1005 (2008).
Elkabets, M. et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J. Clin. Invest. 121, 784–799 (2011).
Kopp, H. G., Ramos, C. A. & Rafii, S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr. Opin. Hematol. 13, 175–181 (2006).
Purhonen, S. et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl Acad. Sci. USA 105, 6620–6625 (2008).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Hattori, K. et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97, 3354–3360 (2001).
DeNardo, D. G. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011).
Anders, H. J., Romagnani, P. & Mantovani, A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol. Med. 20, 154–165 (2014).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Kidd, S. et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27, 2614–2623 (2009).
Melani, C., Chiodoni, C., Forni, G. & Colombo, M. P. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102, 2138–2145 (2003).
Serafini, P., Borrello, I. & Bronte, V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16, 53–65 (2006).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Young, M. R., Wright, M. A., Vellody, K. & Lathers, D. M. Skewed differentiation of bone marrow CD34+ cells of tumor bearers from dendritic toward monocytic cells, and the redirection of differentiation toward dendritic cells by 1alpha, 25-dihydroxyvitamin D3. Int. J. Immunopharmacol. 21, 675–688 (1999).
Yang, L. et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 (2008).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Cortez-Retamozo, V. et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl Acad. Sci. USA 109, 2491–2496 (2012).
Cortez-Retamozo, V. et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38, 296–308 (2013).
Ugurel, S., Rappl, G., Tilgen, W. & Reinhold, U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J. Clin. Oncol. 19, 577–583 (2001).
Holzer, G. et al. Concentration of vascular endothelial growth factor (VEGF) in the serum of patients with malignant bone tumors. Med. Pediatr. Oncol. 36, 601–604 (2001).
Poon, R. T. et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann. Surg. 233, 227–235 (2001).
Van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Rudland, P. S. et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 62, 3417–3427 (2002).
Mor, G. et al. Serum protein markers for early detection of ovarian cancer. Proc. Natl Acad. Sci. USA 102, 7677–7682 (2005).
Tuck, A. B., Chambers, A. F. & Allan, A. L. Osteopontin overexpression in breast cancer: knowledge gained and possible implications for clinical management. J. Cell. Biochem. 102, 859–868 (2007).
Azmi, A. S., Bao, B. & Sarkar, F. H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 32, 623–642 (2013).
Pucci, F. & Pittet, M. J. Molecular pathways: tumor-derived microvesicles and their interactions with immune cells in vivo. Clin. Cancer Res. 19, 2598–2604 (2013).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Grange, C. et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356 (2011).
Fremder, E. et al. Tumor-derived microparticles induce bone marrow-derived cell mobilization and tumor homing: A process regulated by osteopontin. Int. J. Cancer 135, 270–281 (2014).
Zetter, B. R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 49, 407–424 (1998).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Dawson, M. R., Duda, D. G., Chae, S. S., Fukumura, D. & Jain, R. K. VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS ONE 4, e6525 (2009).
Peinado, H., Lavotshkin, S. & Lyden, D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 21, 139–146 (2011).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Sceneay, J. et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 72, 3906–3911 (2012).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Sceneay, J., Smyth, M. J. & Moller, A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 32, 449–464 (2013).
Tsou, C. L. et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909 (2007).
Kang, S. Y. et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc. Natl Acad. Sci. USA 106, 12115–12120 (2009).
Schelter, F. et al. Tumor cell-derived Timp-1 is necessary for maintaining metastasis-promoting Met-signaling via inhibition of Adam-10. Clin. Exp. Metastasis 28, 793–802 (2011).
Jung, T. et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 11, 1093–1105 (2009).
Taranova, A. G. et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 68, 8582–8589 (2008).
Esposito, M. & Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 141, 222–233 (2014).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Roy, L. D. et al. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer 11, 365 (2011).
Naumov, G. N. et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62, 2162–2168 (2002).
Almog, N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 294, 139–146 (2010).
Mullen, C. A., Urban, J. L., Van Waes, C., Rowley, D. A. & Schreiber, H. Multiple cancers. Tumor burden permits the outgrowth of other cancers. J. Exp. Med. 162, 1665–1682 (1985).
Reilly, R. T. et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 60, 3569–3576 (2000).
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011).
Gao, D. et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 72, 1384–1394 (2012).
Castano, Z., Tracy, K. & McAllister, S. S. The tumor macroenvironment and systemic regulation of breast cancer progression. Int. J. Dev. Biol. 55, 889–897 (2011).
Kuznetsov, H. S. et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov. 2, 1150–1165 (2012).
Battinelli, E. M., Markens, B. A. & Italiano, J. E. Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 118, 1359–1369 (2011).
Nilsson, R. J. et al. Blood platelets contain tumor-derived RNA biomarkers. Blood 118, 3680–3683 (2011).
Gay, L. J. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 (2011).
Italiano, J. E. Jr et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111, 1227–1233 (2008).
Labelle, M. & Hynes, R. O. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2, 1091–1099 (2012).
Folkman, J. & Kalluri, R. Cancer without disease. Nature 427, 787 (2004).
Bateman, A. & Bennett, H. P. The granulin gene family: from cancer to dementia. Bioessays 31, 1245–1254 (2009).
Castano, Z. et al. Stromal EGF and IGF1 together modulate plasticity of disseminated triple negative breast tumors. Cancer Discov. 3, 922–935 (2013).
Erez, N., Truitt, M., Olson, P. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Bruzzese, F. et al. Local and systemic pro-tumorigenic effects of fibroblast-derived GDF15/MIC-1. Cancer Res. 74, 3408–3417 (2014).
Breit, S. N. et al. The TGF-beta superfamily cytokine, MIC-1/GDF15: a pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 29, 187–195 (2011).
Li, B. et al. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance-implications for IGF-II and IGF-IR-targeted therapy. Clin. Cancer Res. 20, 2651–2662 (2014).
Lippitz, B. E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 14, e218–228 (2013).
Graus, F. & Dalmau, J. Paraneoplastic neuropathies. Curr. Opin. Neurol. 26, 489–495 (2013).
Taucher, S. et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost. 89, 1098–1106 (2003).
Stone, R. L. et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 366, 610–618 (2012).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Baserga, R., Peruzzi, F. & Reiss, K. The IGF-1 receptor in cancer biology. Int. J. Cancer 107, 873–877 (2003).
Gupta, P. B. et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 67, 2062–2071 (2007).
Demicheli, R., Biganzoli, E., Boracchi, P., Greco, M. & Retsky, M. W. Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res. 10, R83 (2008).
Retsky, M. W., Demicheli, R., Hrushesky, W. J., Baum, M. & Gukas, I. D. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 116, 730–741 (2008).
Gertz, M. A. Current status of stem cell mobilization. Br. J. Haematol. 150, 647–662 (2010).
Ebos, J. M., Lee, C. R., Christensen, J. G., Mutsaers, A. J. & Kerbel, R. S. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc. Natl Acad. Sci. USA 104, 17069–17074 (2007).
Okazaki, T. et al. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int. Immunol. 18, 1–9 (2006).
Shaked, Y. et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 313, 1785–1787 (2006).
Shaked, Y. et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14, 263–273 (2008).
Barcellos-Hoff, M. H. Does microenvironment contribute to the etiology of estrogen receptor-negative breast cancer? Clin. Cancer Res. 19, 541–548 (2013).
Ebos, J. M., Lee, C. R. & Kerbel, R. S. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin. Cancer Res. 15, 5020–5025 (2009).
Guthrie, G. J. et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 88, 218–230 (2013).
Absenger, G. et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br. J. Cancer 109, 395–400 (2013).
Stotz, M. et al. Increased neutrophil–lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer 109, 416–421 (2013).
Ishizuka, M., Nagata, H., Takagi, K., Iwasaki, Y. & Kubota, K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br. J. Cancer 109, 401–407 (2013).
McMillan, D. C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat. Rev. 39, 534–540 (2012).
Acknowledgements
All figures were conceptualized and created by Victor Fanjul (Universidad de Oviedo, Spain; former summer intern in the McAllister lab). We are grateful for the helpful discussions and/or editorial comments of Zvika Granot, Mikael Pittet and Yuval Shaked. S.S.M. is an American Cancer Society Scholar, an AACR Gertrude B. Elion Cancer Research Scholar and a Presidential Early Career Award for Scientists and Engineers scholar. R.A.W. is a Daniel K. Ludwig Professor for Cancer Research at MIT and an American Cancer Society Research Professor. This work was supported in part by grants from the National Institutes of Health (NCI) RO1 CA166284 and the American Cancer Society (S.S.M.); the Breast Cancer Research Foundation (BCRF), National Institutes of Health (NIH), R01 CA078461, P01 CA080111, and the Ludwig Center for Molecular Oncology at MIT (R.A.W.)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McAllister, S., Weinberg, R. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16, 717–727 (2014). https://doi.org/10.1038/ncb3015
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3015
This article is cited by
-
Effect of inflammation on association between cancer and coronary artery disease
BMC Cardiovascular Disorders (2024)
-
TFRC, associated with hypoxia and immune, is a prognostic factor and potential therapeutic target for bladder cancer
European Journal of Medical Research (2024)
-
First-in-human phase I dose escalation trial of the first-in-class tumor microenvironment modulator VT1021 in advanced solid tumors
Communications Medicine (2024)
-
Comparison of ex vivo bioluminescence imaging, Alu-qPCR and histology for the quantification of spontaneous lung and bone metastases in subcutaneous xenograft mouse models
Clinical & Experimental Metastasis (2024)
-
The role of extracellular vesicles in circulating tumor cell-mediated distant metastasis
Molecular Cancer (2023)