Abstract
Microtubule-targeting chemotherapeutics induce apoptosis in cancer cells by promoting the phosphorylation and degradation of the anti-apoptotic BCL-2 family member MCL1. The signalling cascade linking microtubule disruption to MCL1 degradation remains however to be defined. Here, we establish an in vivo screening strategy in Caenorhabditis elegans to uncover genes involved in chemotherapy-induced apoptosis. Using an RNAi-based screen, we identify three genes required for vincristine-induced apoptosis. We show that the DEP domain protein LET-99 acts upstream of the heterotrimeric G protein alpha subunit GPA-11 to control activation of the stress kinase JNK-1. The human homologue of LET-99, DEPDC1, similarly regulates vincristine-induced cell death by promoting JNK-dependent degradation of the BCL-2 family protein MCL1. Collectively, these data uncover an evolutionarily conserved mediator of anti-tubulin drug-induced apoptosis and suggest that DEPDC1 levels could be an additional determinant for therapy response upstream of MCL1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jackson, J. R., Patrick, D. R., Dar, M. M. & Huang, P. S. Targeted anti-mitotic therapies: Can we improve on tubulin agents? Nat. Rev. Cancer 7, 107–117 (2007).
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 (2004).
Jordan, M. A., Thrower, D. & Wilson, L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J. Cell Sci. 102, 401–416 (1992).
Inuzuka, H. et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 (2011).
Wertz, I. E. et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 (2011).
Johnstone, R. W., Ruefli, A. A. & Lowe, S. W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 108, 153–164 (2002).
Horvitz, H. R. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 59, 1701s–1706s (1999).
Gumienny, T. L., Lambie, E., Hartwieg, E., Horvitz, H. R. & Hengartner, M. O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011–1022 (1999).
Gartner, A., Milstein, S., Ahmed, S., Hodgkin, J. & Hengartner, M. O. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5, 435–443 (2000).
Lant, B. & Derry, W. B. Methods for detection and analysis of apoptosis signaling in the C. elegans germline. Methods 61, 174–182 (2013).
Derry, W. B., Putzke, A. P. & Rothman, J. H. Caenorhabditis elegans p53: Role in apoptosis, meiosis, and stress resistance. Science 294, 591–595 (2001).
Conradt, B. & Horvitz, H. R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93, 519–529 (1998).
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001).
Schumacher, B. et al. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 12, 153–161 (2005).
Rieder, C. L. & Maiato, H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7, 637–651 (2004).
Vilpo, J. A., Koski, T. & Vilpo, L. M. Selective toxicity of vincristine against chronic lymphocytic leukemia cells in vitro. Eur. J. Haematol. 65, 370–378 (2000).
Pazdernik, N. & Schedl, T. Introduction to germ cell development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757, 1–16 (2013).
Rocheleau, C. E. et al. The Caenorhabditis elegans ekl (enhancer of ksr-1 lethality) genes include putative components of a germline small RNA pathway. Genetics 178, 1431–1443 (2008).
Siegert, R., Leroux, M. R., Scheufler, C., Hartl, F. U. & Moarefi, I. Structure of the molecular chaperone prefoldin: Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell 103, 621–632 (2000).
Kemphues, K. J., Priess, J. R., Morton, D. G. & Cheng, N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311–320 (1988).
Etemad-Moghadam, B., Guo, S. & Kemphues, K. J. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83, 743–752 (1995).
Wu, J-C. & Rose, L. S. PAR-3 and PAR-1 inhibit LET-99 localization to generate a cortical band important for spindle positioning in Caenorhabditis elegans embryos. Mol. Biol. Cell 18, 4470–4482 (2007).
Rose, L. S. & Kemphues, K. The let-99 gene is required for proper spindle orientation during cleavage of the C. elegans embryo. Development 125, 1337–1346 (1998).
Bringmann, H., Cowan, C. R., Kong, J. & Hyman, A. A. LET-99, GOA-1/GPA-16, and GPR-1/2 are required for aster-positioned cytokinesis. Curr. Biol. 17, 185–191 (2007).
Bellaiche, Y. & Gotta, M. Heterotrimeric G proteins and regulation of size asymmetry during cell division. Curr. Opin. Cell Biol. 17, 658–663 (2005).
Krueger, L. E., Wu, J-C., Tsou, M-F. B. & Rose, L. S. LET-99 inhibits lateral posterior pulling forces during asymmetric spindle elongation in C. elegans embryos. J. Cell Biol. 189, 481–495 (2010).
Tsou, M-F. B., Hayashi, A., DeBella, L. R., McGrath, G. & Rose, L. S. LET-99 determines spindle position and is asymmetrically enriched in response to PAR polarity cues in C. elegans embryos. Development 129, 4469–4481 (2002).
Tsou, M-F. B., Hayashi, A. & Rose, L. S. LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development 130, 5717–5730 (2003).
Chen, S. & Hamm, H. E. DEP domains: More than just membrane anchors. Dev. Cell 11, 436–438 (2006).
Consonni, S. V., Maurice, M. M. & Bos, J. L. DEP domains: Structurally similar but functionally different. Nat. Rev. Mol. Cell Biol. 15, 357–362 (2014).
Ponting, C. P. & Bork, P. Pleckstrin’s repeat performance: A novel domain in G-protein signaling? Trends Biochem. Sci. 21, 245–246 (1996).
Boutros, M., Paricio, N., Strutt, D. I. & Mlodzik, M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94, 109–118 (1998).
Wang, T. H. et al. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J. Biol. Chem. 273, 4928–4936 (1998).
Zhu, B. et al. Activation of Jun N-terminal kinase is a mediator of vincristine-induced apoptosis of melanoma cells. Anticancer Drugs 19, 189–200 (2008).
Vucic, D., Dixit, V. M. & Wertz, I. E. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Biol. 12, 439–452 (2011).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Praitis, V., Casey, E., Collar, D. & Austin, J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217–1226 (2001).
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Follenzi, A. & Naldini, L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 346, 454–465 (2002).
Acknowledgements
We thank Hengartner and Bano laboratory members for help and discussions. We thank L. Rose for the LET-99 antibody. We are grateful to L. Rose and M. Gotta for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation, the Kanton of Zurich and the Josef-Steiner Foundation. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Author information
Authors and Affiliations
Contributions
A.S., X.Z., Y.T., L.S. and S.M.P. performed C. elegans experiments. S.M., C-A.R. and D.B. performed cell culture experiments. D.S., S.M.P., J.M.K., M.S., S.B. and J.N.M. helped with experimental design and procedures. A.S., M.G.M., D.B. and M.O.H. designed experiments. A.S. and M.O.H. wrote the manuscript. All authors provided detailed comments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 Cisplatin, doxorubicin, etoposide and vincristine do not induce cell cycle arrest at the given concentrations.
(a) Synchronized young adult animals were treated with cisplatin, doxorubicin, etoposide, vincristine or ionizing radiation (IR, 60 Gy). Cells per area of the mitotic germline region were quantified 12 h, 24 h and 36 h post-treatment. Ionizing radiation (IR) results in a drop in the number of germ cells in the mitotic region as a result of cell cycle arrest within the mitotic germline compartment. By contrast, chemotherapeutic drugs did not affect the number of mitotic germ cells. Data shown represent the average of three independent experiments ± s.d. (n = 30 quantified germ lines for each time point). (b) PAR-3, LET-99 and PFD-1(C08F8.1) are specifically mediating vincristine-induced germline apoptosis. In contrast, loss of EKL-1 results in hypersensitivity also to IR. Synchronized young adult animals were treated with either IR 60 Gy or UV-C 100 J m−2 and germline apoptosis was quantified 24 h post-treatment. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals for each time point). (c–d) LET-99 mediates apoptosis also in response to other antitubulin chemotherapeutics. Synchronized young adult animals were treated with 0.05 mM nocodazole or 0.05 mM docetaxel and germline apoptosis was quantified 24 h post-treatment. Data represent the average of two independent experiments ± s.d. (n = 40 animals). (e) Vincristine dose-response in the germ line of C. elegans 36 h post-treatment. Data shown represent the average of n = 40 animals from two independent experiments (for 0.1 mM, 0.2 mM and 0.5 mM) or n = 20 (for 0 mM and 0.05 mM) from one experiment ± s.d. (f) Vincristine response in rrf-1(pk1417) mutant animals 36 h post-treatment. Data represent the average of n = 20 animals± s.d. from one experiment. (g) Time course for doxorubicin uptake. Synchronized young adults were treated with doxorubicin 0.1 mM and imaged (doxorubicin fluorescence λex = 470 nm, λem = 590 nm) at the indicated time points. All results are representative images from one experiment. (h–i) The BH3-only domain protein EGL-1 and CED-13 partially mediate vincristine-induced apoptosis. Synchronized young adult animals were exposed to vincristine and germline apoptosis was quantified at the indicated time points. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals for each time point). (j) egl-1 and ced-13 transcripts are induced upon vincristine treatment. Synchronized young adult animals were treated with 0.1 mM vincristine and mRNA levels were evaluated by qRT-PCR. Data shown represent the average ± s.d. from a full 10 cm plate of animals pooled from one experiment.
Supplementary Figure 3 LET-99 localization in embryos and the germ line.
(a–d) LET-99::GFP cortical localization (arrows) in embroys. Scale bars, 10 μm. All results are representative of at least three independent experiments. (e–g) Immunostaining using the LET-99 antibody in wild-type embryos confirms nuclear localization (arrows) of LET-99. Scale bars, 10 μm. (h–l) LET-99 localization does not change in response to vincristine treatment (h-j: LET-99 antibody staining; k-l: LET-99::GFP). Scale bars, 10 μm.
Supplementary Figure 4 An RNAi based screen for G proteins or interactors of LET-99 involved in vincristine-induced germline apoptosis.
(a) Synchronized young adult rrf-1 mutant animals (partially defective in somatic RNAi) were treated with vincristine and germline apoptosis was assessed 36 h post-treatment. Data shown represent the average of n = 20 or n = 40 (gpa-11) animals ± s.d. from one experiment. (b) Synchronized young adult animals were treated with vincristine and germline apoptosis was assessed 36 h following vincristine, 0.1 mM, treatment. Data shown represents the average of n = 20 (control) or n = 30 animals (lin-5, goa-1, gpr-1/2) ± s.d. from one experiment. (c) siRNA directed against DEPDC1A and DEPDC1B attenuates MCL1 degradation also in HeLa cells. Western blot analysis of MCL1 in response to vincristine treatment 12 h and 24 h post-treatment. Results are representative of at least three independent experiments. (d) DEPDC1 does not regulate doxorubicin-induced cell death in HeLa cells. Data shown represent the average ± s.d. from one representative experiment, three independent experiments to assess repeatability (Scramble n = 1741; DEPDC1B n = 1844; DEPDC1A n = 1494; MCL1 n = 1844; Scramble Doxo n = 699; DEPDC1B Doxo n = 782; DEPDC1A Doxo n = 643; Mcl1 Dox n = 507). (e) DEPDC1 mediates vincristine-induced cell death also in SH-SY5Y neuroblastoma cells. Data shown represent the average ± s.d. from one representative experiment, three independent experiments to assess repeatability (Scramble n = 1139; DEPDC1B n = 1168; DEPDC1A n = 1521; Scramble 6 h vin n = 1119; DEPDC1B vin 6 h n = 852; DEPDC1A vin 6 h n = 954; Scramble vin 12 h n = 1115; DEPDC1B vin 12 h n = 989; DEPDC1A vin 12 h n = 1071). (f) Knock-down efficiency by DEPDC1A siRNA and DEPDC1B siRNA versus scramble control siRNA. DEPDC1A and DEPDC1B transcript levels were quantified by qRT-PCR ± s.d. in HeLa and MCF-7 cells treated with siRNA directed either against scramble control siRNA, DEPDC1A siRNA or DEPDC1B siRNA. Data shown are from one experiment, at least three independent experiments to assess repeatability.
Supplementary Figure 5 Multiple alignment of DEPDC1 and LET-99.
(a) Multiple alignment of DEPDC1A (isoforms 1 and 2), DEPDC1B and LET-99. Depicted in red is the DEP domain, in green the RhoGAP domain.
Supplementary Figure 6 The role of JNK-1 in germline apoptosis.
(a) Time course analysis of wild-type and jnk-1(gk7) animals upon IR 60 Gy. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals for each time point). (b) Western blot time-course analysis of phosphorylated JNK-1 upon ionizing radiation (IR) 60 Gy. Results are representative of two independent experiments. (c) The MAP kinase kinases MEK-1 and SEK-1 are dispensable for JNK-1 phosphorylation upon vincristine treatment. Western blot analysis of phosphorylated JNK-1 in synchronized mek-1(ks54) and sek-1(km4) animals 6 h post vincristine treatment (0.1 mM). Results are representative of two independent experiments. (d) Time-course analysis of wild-type and mkk-4(km23) animals upon vincristine treatment. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals for each time point). (e) MKK-4 is required for UV-C-induced apoptosis. Synchronized wild-type and mkk-4(km23) animals were treated with UV-C or IR and germ cell apoptosis was quantified 24 h post-treatment. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals). (f–h) Time-course analysis of wild-type and jnk-1(gk7) animals following chemotherapy treatment. Data shown represent the average of three independent experiments ± s.d. (n = 60 animals for each time point). (i) Western blot analysis of phosphorylated JNK in MCF-7 cells. Results are representative of at least three independent experiments.
Supplementary Figure 7 Uncropped images of blots.
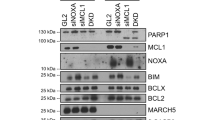
Uncropped versions of key electrophoretic data have been provided. These scans correspond to data in Figs 3d–f, 4h–i, 5a, b, d, h, and Supplementary Fig. 3c.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2060 kb)
Rights and permissions
About this article
Cite this article
Sendoel, A., Maida, S., Zheng, X. et al. DEPDC1/LET-99 participates in an evolutionarily conserved pathway for anti-tubulin drug-induced apoptosis. Nat Cell Biol 16, 812–820 (2014). https://doi.org/10.1038/ncb3010
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3010
This article is cited by
-
DEPDC1 and KIF4A synergistically inhibit the malignant biological behavior of osteosarcoma cells through Hippo signaling pathway
Journal of Orthopaedic Surgery and Research (2023)
-
Silencing eL31 suppresses the progression of colorectal cancer via targeting DEPDC1
Journal of Translational Medicine (2022)
-
MINA-1 and WAGO-4 are part of regulatory network coordinating germ cell death and RNAi in C. elegans
Cell Death & Differentiation (2019)
-
UNG-1 and APN-1 are the major enzymes to efficiently repair 5-hydroxymethyluracil DNA lesions in C. elegans
Scientific Reports (2018)
-
WAH-1/AIF regulates mitochondrial oxidative phosphorylation in the nematode Caenorhabditis elegans
Cell Death Discovery (2018)