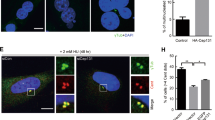
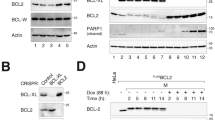
Abstract
Cyclins B1 and B2 are frequently elevated in human cancers and are associated with tumour aggressiveness and poor clinical outcome; however, whether and how B-type cyclins drive tumorigenesis is unknown. Here we show that cyclin B1 and B2 transgenic mice are highly prone to tumours, including tumour types where B-type cyclins serve as prognosticators. Cyclins B1 and B2 both induce aneuploidy when overexpressed but through distinct mechanisms, with cyclin B1 inhibiting separase activation, leading to anaphase bridges, and cyclin B2 triggering aurora-A-mediated Plk1 hyperactivation, resulting in accelerated centrosome separation and lagging chromosomes. Complementary experiments revealed that cyclin B2 and p53 act antagonistically to control aurora-A-mediated centrosome splitting and accurate chromosome segregation in normal cells. These data demonstrate a causative link between B-type cyclin overexpression and tumour pathophysiology, and uncover previously unknown functions of cyclin B2 and p53 in centrosome separation that may be perturbed in many human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 September 2014
In the version of this Article originally published, the authors incorrectly cited ref. 39, and this has now been deleted from the online versions of the Article. In addition, the authors omitted four key references, which have now been added as below: In the following sentences on page 540: "Plk1 activity typically increases in late G2, resulting in phosphorylation and activation of Mst2, which in turn phosphorylates and activates Nek2A67,68. Activated Nek2A then phosphorylates C-Nap1 and rootletin, two proteins that hold duplicated centrosomes, resulting in centrosome separation69,70". In the legend of Fig. 5j, as well as an additional citation of ref. 38: "Cyclin B2–Cdk1, on the other hand, triggers the phosphorylation of the residual aurora A protein pool in a timely manner to induce phosphorylation of centrosome-associated Plk1 and permit centrosome disjunction38,67–70". Additional references: 67. Mardin, B. R. et al. Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat. Cell Biol. 12, 1166–1176 (2010). 68. Mardin, B. R., Agircan, F. G., Lange, C. & Schiebel, E. Plk1 controls the Nek2A-PP1γ antagonism in centrosome disjunction. Curr. Biol. 21, 1145–1151 (2011). 69. Fry, A. M.et al. C Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141, 1563–1574 (1998). 70. Bahe, S., Stierhof, Y. D., Wilkinson, C. J., Leiss, F. & Nigg, E. A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 171, 27–33 (2005).
References
Holland, A. J. & Cleveland, D. W. Losing balance: The origin and impact of aneuploidy in cancer. EMBO Rep. 13, 501–514 (2012).
Pfau, S. J. & Amon, A. Chromosomal instability and aneuploidy in cancer: From yeast to man. EMBO Rep. 13, 515–527 (2012).
Schvartzman, J. M., Sotillo, R. & Benezra, R. Mitotic chromosomal instability and cancer: Mouse modelling of the human disease. Nat. Rev. Cancer 10, 102–115 (2010).
Stirling, P. C. et al. The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet. 7, e1002057 (2011).
McGranahan, N., Burrell, R. A., Endesfelder, D., Novelli, M. R. & Swanton, C. Cancer chromosomal instability: Therapeutic and diagnostic challenges. EMBO Rep. 13, 528–538 (2012).
Ricke, R. M. & van Deursen, J. M. Aneuploidy in health, disease and aging. J. Cell Biol. 201, 11–21 (2013).
Glinsky, G. V., Berezovska, O. & Glinskii, A. B. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J. Clin. Invest. 115, 1503–1521 (2005).
Parris, T. Z. et al. Clinical implications of gene dosage and gene expression patterns in diploid breast carcinoma. Clin. Cancer Res. 16, 3860–3874 (2010).
Nakagawa, T. et al. A tissue biomarker panel predicting systemic progression after PSA recurrence post-definitive prostate cancer therapy. PLoS One 3, e2318 (2008).
Carter, S. L., Eklund, A. C., Kohane, I. S., Harris, L. N. & Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 38, 1043–1048 (2006).
Chapman, D. L. & Wolgemuth, D. J. Isolation of the murine cyclin B2 cDNA and characterization of the lineage and temporal specificity of expression of the B1 and B2 cyclins during oogenesis, spermatogenesis and early embryogenesis. Development 118, 229–240 (1993).
Draviam, V. M., Orrechia, S., Lowe, M., Pardi, R. & Pines, J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 152, 945–958 (2001).
Pines, J. & Hunter, T. The differential localization of human cyclins A and B is due to a cytoplasmic retention signal in cyclin B. EMBO J. 13, 3772–3781 (1994).
Jackman, M., Firth, M. & Pines, J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 14, 1646–1654 (1995).
Brandeis, M. et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl Acad. Sci. USA 95, 4344–4349 (1998).
Bailly, E., Pines, J., Hunter, T. & Bornens, M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J. Cell Sci. 101 (Pt 3), 529–545 (1992).
Pines, J. Cyclins: wheels within wheels. Cell Growth Differ. 2, 305–310 (1991).
Nigg, E. A. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol. 3, 296–301 (1993).
Gavet, O. & Pines, J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533–543 (2010).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 (2006).
Spalluto, C., Wilson, D. I. & Hearn, T. Evidence for centriolar satellite localization of CDK1 and cyclin B2. Cell Cycle 12, 1802–1803 (2013).
Yoshitome, S., Furuno, N., Hashimoto, E. & Sagata, N. The C-terminal seven amino acids in the cytoplasmic retention signal region of cyclin B2 are required for normal bipolar spindle formation in Xenopus oocytes and embryos. Mol. Cancer Res. 1, 589–597 (2003).
Yoshitome, S., Furuno, N. & Sagata, N. Overexpression of the cytoplasmic retention signal region of cyclin B2, but not of cyclin B1, inhibits bipolar spindle formation in Xenopus oocytes. Biol. Cell. 90, 509–518 (1998).
Kotani, T., Yoshida, N., Mita, K. & Yamashita, M. Requirement of cyclin B2, but not cyclin B1, for bipolar spindle formation in frog (Rana japonica) oocytes. Mol. Reprod. Dev. 59, 199–208 (2001).
Beard, C., Hochedlinger, K., Plath, K., Wutz, A. & Jaenisch, R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 44, 23–28 (2006).
Hochedlinger, K., Yamada, Y., Beard, C. & Jaenisch, R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477 (2005).
Kumada, K. et al. The selective continued linkage of centromeres from mitosis to interphase in the absence of mammalian separase. J. Cell Biol. 172, 835–846 (2006).
Wirth, K.G. et al. Separase: A universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172, 847–860 (2006).
Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P. & Kirschner, M. W. Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 (2001).
Kawabata, T. et al. Stalled fork rescue via dormant replication origins in unchallenged S phase promotes proper chromosome segregation and tumor suppression. Mol. Cell 41, 543–553 (2011).
Dawlaty, M. M. et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIα. Cell 133, 103–115 (2008).
Jeganathan, K., Malureanu, L., Baker, D. J., Abraham, S. C. & van Deursen, J. M. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J. Cell Biol. 179, 255–267 (2007).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Silkworth, W. T., Nardi, I. K., Scholl, L. M. & Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One 4, e6564 (2009).
Zhang, Y. et al. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J. Clin. Invest. 122, 4362–4374 (2012).
Kabeche, L. & Compton, D. A. Checkpoint-independent stabilization of kinetochore-microtubule attachments by Mad2 in human cells. Curr. Biol. 22, 638–644 (2012).
Silkworth, W. T. & Cimini, D. Transient defects of mitotic spindle geometry and chromosome segregation errors. Cell Div. 7, 19–26 (2012).
Bruinsma, W., Raaijmakers, J. A. & Medema, R. H. Switching Polo-like kinase-1 on and off in time and space. Trends Biochem. Sci. 37, 534–542 (2012).
Smith, E. et al. Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. Embo J. 30, 2233–2245 (2011).
Chen, Y., Yeh, P. C., Huang, J. C., Yeh, C. C. & Juang, Y. L. The spindle checkpoint protein MAD1 regulates the expression of E-cadherin and prevents cell migration. Oncol. Rep. 27, 487–491 (2012).
Macurek, L. et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455, 119–123 (2008).
Seki, A., Coppinger, J. A., Jang, C. Y., Yates, J. R. & Fang, G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658 (2008).
Carmena, M. et al. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol. 10, e1001250 (2012).
Kiyomitsu, T. & Cheeseman, I. M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat. Cell Biol. 14, 311–317 (2012).
Suijkerbuijk, S. J., Vleugel, M., Teixeira, A. & Kops, G. J. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev. Cell 23, 745–755 (2012).
Hahn, W. C. et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell Biol. 22, 2111–2123 (2002).
Wu, C. C. et al. p53 negatively regulates Aurora A via both transcriptional and posttranslational regulation. Cell Cycle 11, 3433–3442 (2012).
Soria, J. C. et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 60, 4000–4004 (2000).
Yoshida, T., Tanaka, S., Mogi, A., Shitara, Y. & Kuwano, H. The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann. Oncol.: Official J. Eur. Soc. Med. Oncol./ESMO 15, 252–256 (2004).
Cooper, W. A. et al. Expression and prognostic significance of cyclin B1 and cyclin A in non-small cell lung cancer. Histopathology 55, 28–36 (2009).
Park, S. H. et al. NF-Y-dependent cyclin B2 expression in colorectal adenocarcinoma. Clin. Cancer Res. 13, 858–867 (2007).
Wang, A., Yoshimi, N., Ino, N., Tanaka, T. & Mori, H. Overexpression of cyclin B1 in human colorectal cancers. J. Cancer Res. Clin. Oncol. 123, 124–127 (1997).
Sarafan-Vasseur, N. et al. Overexpression of B-type cyclins alters chromosomal segregation. Oncogene 21, 2051–2057 (2002).
Dove, W. F. et al. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 915–923 (1998).
Moser, M. J. et al. Genetic instability and hematologic disease risk in Werner syndrome patients and heterozygotes. Cancer Res. 60, 2492–2496 (2000).
Babu, J. R. et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J. Cell Biol. 160, 341–353 (2003).
Van Ree, J. H., Jeganathan, K. B., Malureanu, L. & van Deursen, J. M. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J. Cell Biol. 188, 83–100 (2010).
Kasper, L. H. et al. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol. Cell Biol. 19, 764–776 (1999).
Hart, P. E., Glantz, J. N., Orth, J. D., Poynter, G. M. & Salisbury, J. L. Testis-specific murine centrin, Cetn1: genomic characterization and evidence for retroposition of a gene encoding a centrosome protein. Genomics 60, 111–120 (1999).
Baker, D. J., Jin, F., Jeganathan, K. B. & van Deursen, J. M. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 16, 475–486 (2009).
Jeganathan, K., Malureanu, L., Baker, D. J., Abraham, S. C. & van Deursen, J. M. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J. Cell Biol. 179, 255–267 (2007).
Malureanu, L. et al. Cdc20 hypomorphic mice fail to counteract de novo synthesis of cyclin B1 in mitosis. J. Cell Biol. 191, 313–329 (2010).
Sakaue-Sawano, A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008).
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 11, 27–35 (2009).
McKenzie, L. et al. p53-dependent repression of polo-like kinase-1 (PLK1). Cell Cycle 9, 4200–4212 (2010).
Mardin, B. R. et al. Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat. Cell Biol. 12, 1166–1176 (2010).
Mardin, B. R., Agircan, F. G., Lange, C., Schiebel, E. Plk1 controls the Nek2A-PP1γ antagonism in centrosome disjunction. Curr. Biol. 21, 1145–1151 (2011).
Fry, A. M. et al. Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141, 1563–1574 (1998).
Bahe, S., Stierhof, Y. D., Wilkinson, C. J., Leiss, F., Nigg, E. A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 171, 27–33 (2005).
Acknowledgements
We thank W. Zhou and M. Li of Mayo Clinic’s Gene Knockout Mouse Core Facility for ES cell microinjection and chimaera breeding, and L. Malureanu, K. Jeganathan and D. Baker for help with live-cell imaging, karyotyping and tumour analyses, respectively. We are grateful to P. Galardy, R. Ricke, R. Naylor, D. Baker and L. Malureanu for discussions and critical evaluation of this manuscript, J. Salisbury for sharing antibody, J-M. Peters for sharing the Scc1 construct, D. Compton and L. Kabeche for sharing photoactivable GFP–α-tubulin construct and help with measurements of microtubule dynamics, V. Shridhar for human lung (tumour) samples and the Mayo Clinic Cytogenetics shared resource for FISH analysis. This work was supported by the National Institutes of Health (CA126828 to J.M.v.D.).
Author information
Authors and Affiliations
Contributions
H-J.N. and J.M.v.D. conceived the research and designed experiments. H-J.N. carried out experiments and analysed results. H-J.N. and J.M.v.D. discussed results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Analysis of CcnB1 and CcnB2 transgene expression.
(a and b) Western blot analysis of tissue extracts from 6-week-old dox-treated HA-CcnB1 (strains T7 and T11), HA-CcnB2 (strains T15 and T16) and control (TA) transgenic mice. Ponceau S staining of blotted proteins served as a loading control (c) Immunoblots of tissue extracts from 6-week-old dox-treated and untreated HA-CcnB1T7 or HA-CcnB2T16 transgenic mice demonstrating that transgene expression is tightly controlled by dox. All blots are representative of two independent experiments.
Supplementary Figure 2 Cyclin B1 protein levels and Cdk substrate phosphorylation are increased in CcnB1 transgenic MEFs during mitosis.
(a) Dox-treated and untreated CcnB1T7 MEFs at various stages of cell cycle progression stained for cyclin B1. P-H3S10 staining was used for cell cycle staging. (b) Quantification of cyclin B1 signals of dox-treated and untreated CcnB1T7 MEFs at various stages of the cell cycle. (c) As in a but stained for phosphorylated Cdk substrates. γ-Tubulin staining was used for cell cycle staging. (d) Quantification of pCdk substrate signals of dox-treated and untreated CcnB1T7 MEFs at various stages of the cell cycle. DNA was visualized with Hoechst. Scale bars, 10 μm. Data in b and d represent mean ± s.e.m. (per group, we analysed ≥10 cells in b, and ≥8 cells in d). Statistical significance was determined by a two-tailed, unpaired t-test. Statistics source data are provided in Supplementary Table 2. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 3 Cyclin B2 protein levels and Cdk substrate phosphorylation are increased in CcnB2 transgenic MEFs throughout the cell cycle.
(a) Dox-treated and untreated CcnB2T16 MEFs at various stages of cell cycle progression stained for cyclin B2. γ-Tubulin staining was used for cell cycle staging. (b) Quantification of cyclin B2 signals of dox-treated and untreated CcnB2T16 MEFs at various stages of the cell cycle. (c) As in a but stained for phosphorylated Cdk substrates. γ-Tubulin staining was used for cell cycle staging. (d) Quantification of pCdk substrate signals of dox-treated and untreated CcnB2T16 MEFs at various stages of the cell cycle. DNA was visualized with Hoechst. Scale bars, 10 μm. Data in b and d represent mean ± s.e.m. (per group, we analysed ≥8 cells in b and d). Statistical significance was determined by two-tailed, unpaired t-test. Statistics source data are provided in Supplementary Table 2. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 4 Cyclin B1 overexpression does not appear to disrupt early mitosis.
(a) Quantification of total cyclin B1 signal in dox-treated CcnB1T7 anaphases with and without chromatin bridges. Data represent mean ± s.e.m. Three independent MEF lines were included in the analysis. (b) Time-lapse images of biosensor-positive CcnB2T16 MEFs progressing through mitosis in the absence or presence of transgenic cyclin B2. Bars, 5 μm. (c) Quantification of biosensor fluorescence at chromosomes as cells progress through mitosis. Data represent mean ± s.e.m. from five independent experiments. (d) Western blot analysis of lysates from induced and non-induced CcnB1T7 MEFs for Top2a (blots are representative of two independent experiments). (e) Images of CcnB1T7 prometaphases immunostained for centromeres and Top2a. Insets show enlarged centromeres from boxed region. Bar, 5 μm. (f and g) Quantitation of RPA2- or γH2AX/53BP1-positive foci in induced and non-induced CcnB1T7 and CcnB2T16 MEFs. Data represent mean ± s.e.m. from three independent MEF lines (we analysed ≥100 cells per line). (h) Timing of early stages of mitosis of induced and non-induced CcnB1T7 MEFs by live-cell imaging. P, prophase; PM, prometaphase; M, metaphase; and A, anaphase onset. Data represent mean ± s.e.m. from three independent MEF lines (we analysed 10 cells per line) (i) Analysis of S/G2 duration in induced and non-induced CcnB1T7 MEFs using FUCCI technology. Data represent mean ± s.e.m. from three independent MEF lines (we analysed ≥10 cells per line). (j) Growth curves of induced and non-induced CcnB1T7 and CcnB2T16 MEFs (P3). Data represent mean ± s.e.m. from three independent MEF lines. *P < 0.05 denotes a statically significant cell number at day 5. Statistical significance was determined by a two-tailed unpaired t-test. Statistics source data are provided in Supplementary Table 2.
Supplementary Figure 5 Characterization of the chromosome lagging phenotype of cyclin B2 overexpressing cells.
(a) Nocodazole- and taxol-challenge assays on CcnB1T7 and CcnB2T16 MEFs with or without transgene induction. Data represent mean ± s.e.m. from three independent MEF lines (we analysed ≥10 cells per line). (b) Quantification of cells with misaligned chromosomes following release from monastrol and MG132 exposure. Data represent mean ± s.e.m. from three independent MEF lines (we analysed ≥25 cells per line). (c) Measurement of kinetochore-microtubule dynamics in cyclin B2 transgenic MEFs. Normalized fluorescence intensity over time after photoactivation of spindles in prometaphase cells. Data represent mean ± s.e.m. of 8 cells per genotype. (d and e) Images of methanol-fixed CcnB2T16 MEFs immunostained for the centrosome marker Centrin 2 and either cyclin B2 (d) or HA (e). DNA was visualized with Hoechst. Scale bar, 10 μm. (f) Dox-treated CcnB2T16 MEFs in G2 analysed for centrosome distance and level of HA cyclin B2 expression (29 cells were analysed). Note that there is a correlation between level of overexpression and centrosome distance. (g) Accelerated centrosome separation at various concentrations of Plk1 inhibitor. Data represent mean ± s.e.m. from three independent MEF lines (we analysed 20 cells per line). Cells with centrosome distances ≤8 μm were classified as accelerated. (h) Quantitation of p-Aurora-A signals at centrosomes at the various stages of mitosis. Data represent mean ± s.e.m. of 7–11 cells per group. (i) Quantitation of p-Plk1T210 signal at centrosomes in G2 CcnB2T16 MEFs (a.u., arbitrary unit). Data represent mean ± s.e.m. from three independent MEF lines (we analysed 10 cells per line). (j) Incidence of spindle geometry abnormalities at various concentrations of Aurora A inhibitor MLN8054. Data represent mean ± s.e.m. from three independent MEF lines (we analysed 20 cells per line). (k) Images of CcnB2T16 prometaphases immunostained for p-Plk1T210, p-BubR1T680, centromeres. p-BubR1T680 phosphorylation by p-Plk1T210 mediates recruitment of PP2A to kinetochores to prevent uncontrolled Aurora B activity and destabilization of microtubule-kinetochore attachments. DNA was visualized with Hoechst. Bar, 10 μm. (l) Quantification of p-BubR1T680 signal in presence or absence of elevated cyclin B2. Note that prometaphases accumulate normal levels of p-BubR1T680, indicating that attachment error correction at kinetochores is unperturbed when cyclin B2 levels are upregulated. Data represent mean of 10 cells per group. (m) Spindle position measurements. Data represent mean ± s.d. of 20 cells per group. Statistical significance for was determined by a two-tailed, unpaired t-test. Statistics source data are provided in Supplementary Table 2.
Supplementary Figure 6 p53 loss increases Aurora A and Plk1 transcript and protein levels.
(a) Western blots of extracts from wild-type and p53−/− MEFs. Actin probe and Ponceau S staining served as loading controls (blots are representative of two independent experiments). (b) Aurka and Plk1 transcript levels in wild-type and p53/MEFs analysed by qRT-PCR. Data represent mean ± s.e.m. from three independent experiments (we measured duplicate of two independent MEF lines per experiment). GAPDH transcript levels were used for normalization. Values were normalized against wild-type values. Consistent with evidence that p53 mediates transcriptional repression of Plk166, we find that Plk1 is upregulated in p53−/− MEFs. However, it is important to emphasize that even though Plk1 expression is upregulated, Plk1 hyperactivity in p53−/− MEFs remains fully dependent on Aurora A hyperactivity as low amounts of Aurora A kinase MLN8054 inhibitor restore proper timing of centrosome separation, spindle geometry, and accurate chromosome segregation (Fig. 5g–i). (c) Quantitation of p-Aurora A intensity at centrosomes in G2 cells. (d) Quantitation of p-Plk1T210 intensity at centrosomes G2 cells. Data in c and d represent mean ± s.e.m. from three independent MEF lines (we analysed 10 cells per line). Statistical significance was determined by a two-tailed, unpaired t-test. Statistics source data are provided in Supplementary Table 2. *P < 0.05, ***P < 0.001.
Supplementary Figure 7 CCNB1 and CCNB2 are frequently overexpressed in human lung carcinomas.
CCNB1 (a), CCNB2 (b), Ki67 (c) and BUB1 (d) transcript levels in normal human lung tissue, lung adenocarcinomas, and squamous cell lung carcinomas analysed by qRT-PCR. Data represent the mean of duplicate measurements. TBP transcript levels were used for normalization. The relative fold CCNB1, CCNB2, Ki67 or BUB1 transcript in tumors was normalized to the average of the normal lung tissue values. Ki67 and BUB1 transcripts were used as proliferation and mitotic markers, respectively.
Supplementary Figure 8 Quantitation of small intestinal tumors and colon tumor histopathology 30 day after dox withdrawal.
(a) Tumor multiplicity in small intestine. n represents the number of mice analysed. Data represent mean ± s.e.m. Statistical significance was determined by two-tailed unpaired t-test. (b) Hematoxylin-eosin-stained sections of colonic polyps of mice that were taken off dox for the last 30 days of the experiment. Black and red scale bars are 2 mm and 100 μm, respectively.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2530 kb)
Supplementary Table 1
Supplementary Information (XLSX 47 kb)
Supplementary Table 2
Supplementary Information (XLSX 143 kb)
Rights and permissions
About this article
Cite this article
Nam, HJ., van Deursen, J. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol 16, 535–546 (2014). https://doi.org/10.1038/ncb2952
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2952
This article is cited by
-
Upregulation of CCNB2 and a novel lncRNAs-related risk model predict prognosis in clear cell renal cell carcinoma
Journal of Cancer Research and Clinical Oncology (2024)
-
Twenty years of merotelic kinetochore attachments: a historical perspective
Chromosome Research (2023)
-
Identification and verification of CCNB1 as a potential prognostic biomarker by comprehensive analysis
Scientific Reports (2022)
-
Murine allele and transgene symbols: ensuring unique, concise, and informative nomenclature
Mammalian Genome (2022)
-
Screening and predicted value of potential biomarkers for breast cancer using bioinformatics analysis
Scientific Reports (2021)