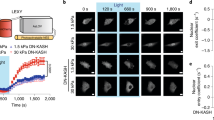
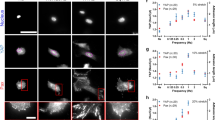
Abstract
Mechanical forces influence many aspects of cell behaviour. Forces are detected and transduced into biochemical signals by force-bearing molecular elements located at the cell surface, in adhesion complexes or in cytoskeletal structures1. The nucleus is physically connected to the cell surface through the cytoskeleton and the linker of nucleoskeleton and cytoskeleton (LINC) complex, allowing rapid mechanical stress transmission from adhesions to the nucleus2. Although it has been demonstrated that nuclei experience force3, the direct effect of force on the nucleus is not known. Here we show that isolated nuclei are able to respond to force by adjusting their stiffness to resist the applied tension. Using magnetic tweezers, we found that applying force on nesprin-1 triggers nuclear stiffening that does not involve chromatin or nuclear actin, but requires an intact nuclear lamina and emerin, a protein of the inner nuclear membrane. Emerin becomes tyrosine phosphorylated in response to force and mediates the nuclear mechanical response to tension. Our results demonstrate that mechanotransduction is not restricted to cell surface receptors and adhesions but can occur in the nucleus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 (2009).
Maniotis, A. J., Chen, C. S. & Ingber, D. E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA 94, 849–854 (1997).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Matthews, B. D., Overby, D. R., Mannix, R. & Ingber, D. E. Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 119, 508–518 (2006).
Choquet, D., Felsenfeld, D. P. & Sheetz, M. P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39–48 (1997).
Guilluy, C. et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 724–729 (2011).
Hofmann, W. A. & de Lanerolle, P. Nuclear actin: To polymerize or not to polymerize. J. Cell Biol. 172, 495–496 (2006).
Dubash, A. D. et al. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PloS ONE 6, e17380 (2011).
Dahl, K. N. & Kalinowski, A. Nucleoskeleton mechanics at a glance. J. Cell Sci. 124, 675–678 (2011).
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J. & Discher, D. E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104, 15619–15624 (2007).
Lammerding, J. et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370–378 (2004).
Sosa, B. A., Rothballer, A., Kutay, U. & Schwartz, T. U. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035–1047 (2012).
Lei, K. et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl Acad. Sci. USA 106, 10207–10212 (2009).
Lammerding, J. et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 170, 781–791 (2005).
Rowat, A. C., Lammerding, J. & Ipsen, J. H. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys. J. 91, 4649–4664 (2006).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006).
Takahashi, A. et al. Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp. Cell Res. 315, 1117–1141 (2009).
Chu, I. et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128, 281–294 (2007).
Taagepera, S. et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl Acad. Sci. USA 95, 7457–7462 (1998).
Lim, S. T. et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 29, 9–22 (2008).
Tifft, K. E., Bradbury, K. A. & Wilson, K. L. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J. Cell Sci. 122, 3780–3790 (2009).
Ho, C. Y. & Lammerding, J. Lamins at a glance. J. Cell Sci. 125, 2087–2093 (2012).
Provenzano, P. P. & Keely, P. J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 124, 1195–1205 (2011).
Khatau, S. B. et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl Acad. Sci. USA 106, 19017–19022 (2009).
Luxton, G. W., Gomes, E. R., Folker, E. S., Vintinner, E. & Gundersen, G. G. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 329, 956–959 (2010).
Folker, E. S., Ostlund, C., Luxton, G. W., Worman, H. J. & Gundersen, G. G. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc. Natl Acad. Sci. USA 108, 131–136 (2011).
Khatau, S. B. et al. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci. Rep. 2, 1–11 (2012).
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K. & Lammerding, J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511 (2013).
Friedl, P., Wolf, K. & Lammerding, J. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23, 55–64 (2011).
Philip, J. T. & Dahl, K. N. Nuclear mechanotransduction: Response of the lamina to extracellular stress with implications in aging. J. Biomechanics 41, 3164–3170 (2008).
Fisher, J. K. et al. Thin-foil magnetic force system for high-numerical-aperture microscopy. Rev. Sci. Instrum. 77, nihms8302 (2006).
Guilluy, C. et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 724–729 (2011).
Ren, X. D., Kiosses, W. B. & Schwartz, M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Acknowledgements
This study was supported by National Institutes of Health Grants numbers GM029860 (to K.B.), P41-EB002025-23A1 (R.S.) and R01-HL077546-03A2 (R.S.), and a grant from the University Cancer Research Fund from the Lineberger Comprehensive Cancer Center. C.G. is supported by a Marie Curie Outgoing International Fellowship from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 254747.
Author information
Authors and Affiliations
Contributions
C.G. designed and performed experiments. L.S., L.D.O., L.V.L., R.S. and R.G-M. helped with experimental design and procedures. C.G. and K.B. wrote the manuscript. K.B. directed the project. All authors provided detailed comments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Nuclear stiffening in response to force applied to nesprin-1.
Typical displacement plot for control sh (a—left panel), emerin sh2(b) and laminA/C sh1 (c). Displacement curve transformed into compliance with Jeffreys model fit (a—right panel) (Jeffreys model description in Supplementary Fig. 2a).
Supplementary Figure 2 Viscoelastic behavior of the nucleus.
To examine the stiffness of the nucleus in response to an applied force, the time-dependent compliance of the nucleus was calculated from the time-dependent displacement using:J(t) = 6πax(t)/F(t), where a is the bead radius. a, The viscoelastic response of the nucleus was characterized by fitting the compliance during force application to a Jeffreys model, a mechanical circuit model used to describe viscoelastic material. The Jeffreys model is formed by an elastic spring and viscous dashpot in parallel with a dashpot in series. b, Example bead displacement curve. c, Example bead displacement curve transformed into compliance with overlay of Jeffreys model fit.
Supplementary Figure 3 Characterization of the nuclear stiffening in response to force.
a, Nuclei isolated from Hela cells were incubated with anti-nesprin-1-coated magnetic beads and stimulated with a permanent magnet for different amounts of time. Active RhoA (RhoAGTP) was isolated with GSTRBD (Rho-binding domain) and analysed by western blotting. All results are representative of at least three independent experiments. b, Stable cell lines depleted for Lamin A/C, LAP2α, emerin, SUN1 or SUN2 were generated using shRNA. Efficiency of the knockdown was assessed by western blot. All results are representative of at least three independent experiments. c, Change in bead displacement between the first and the 6th pulse of force applied to beads coated with anti-nesprin-1 antibody bound to nuclei isolated from cells transfected with control siRNA (n = 12 beads) for MAN1 siRNA (n = 15 beads) and LBR siRNA (n = 17 beads). Displacements were calculated relative to the first pulse of force (error bars represent s.e.m., *P < 0.05, data were collected from 3 independent experiments and analysed by two-tailed unpaired t-test). d, Efficiency of siRNA knockdowns for MAN1 and LBR were assessed by w
Supplementary Figure 4 SFK(s) mediate the nuclear stiffening to force.
a, Nuclei isolated from Hela cells were incubated with anti-nesprin-1-coated magnetic beads and stimulated with a permanent magnet for different amounts of time. Emerin was immunoprecipitated and its tyrosine phosphorylation was analysed by western blot. All results are representative of at least three independent experiments. b, Nuclei isolated from Hela cells were incubated with anti-nesprin-1-coated magnetic beads and pretreated 30 min with Gleevec (10 μM), SU66056 (2.5 μM) or FAK inhibitor (5 μM). After stimulation with a permanent magnet for 3 min, tyrosine phosphorylation of nuclear proteins was analysed by western blot. All results are representative of at least three independent experiments. c, Nuclei isolated from Hela cells were incubated with anti-nesprin-1-coated magnetic beads and stimulated with a permanent magnet for 3 min. Src expression, Src phosphorylation on Y416, FAK expression and FAK phosphorylation on Y397 were assessed by western blot. All results are representative of at least three independent experiments. d, Change in bead displacement between the first and 6th pulse of force applied to beads coated with anti-nesprin-1 antibody bound to nuclei incubated with no ATP (n = 14 beads) or with SU6656 (n = 22 beads) for 30 min (untreated control n = 13 beads). Displacements were calculated relative to the first pulse of force applied to untreated nuclei (Error bars represent s.e.m., *P < 0.05, data were collected from 3 independent experiments and analysed by two-tailed unpaired t-test).
Supplementary Figure 5 Tension induces emerin phosphorylation.
a, Emerin tyrosine phosphorylation was analysed after immunoprecipitation in MRC5 cells during adhesion to fibronectin or treated with blebbistatin. (‘total’ refers to the emerin level in nuclear lysates). All results are representative of at least three independent experiments. b, MRC5 cells were incubated with fibronectin-coated magnetic beads for 30 min. A permanent magnet was used to generate tensional force for different amounts of time. After cell lysis, emerin tyrosine phosphorylation was analysed. All results are representative of at least three independent experiments. c, Invasion of emerin knockdown Hela cells and emerin knockdown Hela cells re-expressing WT or 74-95FF emerin mutant was evaluated by Transwell migration assays. Cells were plated in the upper chamber of the filters and after 8 h cells that had migrated to the underside of the filters were fixed. Relative cell migration was determined by the number of cells that had migrated to the underside of the filter normalized to the total number of cells. A number of n = 24 fields were observed per condition. The value from control shRNA Hela cells was arbitrarily set at 100% (Error bars represent s.e.m., #P < 0.05 compared to control sh, *P < 0.05 compared to WT, data were collected from 4 independent experiments and analysed by one way ANOVA). d, Emerin knockdown MRC5 cells re-expressing WT or 74-95FF emerin mutant were grown on fibronectin-coated coverslips for 6 h, fixed, permeabilized and stained for YAP/TAZ and myc tagged emerin. To quantify YAP or TAZ nuclear localization (panel d) we calculated the percentage of cells with a predominant nuclear staining (delimited by DAPI staining) among the total cell number. n = 36 myc positive cells expressing WT emerin and n = 34 myc positive cells expressing 74-95FF were analysed (Error bars represent s.e.m., *P < 0.05, data were collected from 3 independent experiments and analysed by two-tailed unpaired t-test). Bar scale = 25 μm. e, Emerin knockdown MRC5 cells re-expressing WT or 74-95FF emerin mutant were incubated with fibronectin-coated magnetic beads for 20 min. A permanent magnet was used to generate tensional force for 90 min. IEX1 and GAPDH mRNA levels were analysed using real-time qPCR (error bars represent s.e.m., #P < 0.05 compared to WT control, data were collected from n = 3 independent experiments and analysed by two-tailed unpaired t-test).
Supplementary information
Supplementary Information
Supplementary Information (PDF 661 kb)
Rights and permissions
About this article
Cite this article
Guilluy, C., Osborne, L., Van Landeghem, L. et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16, 376–381 (2014). https://doi.org/10.1038/ncb2927
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2927
This article is cited by
-
Modelling and targeting mechanical forces in organ fibrosis
Nature Reviews Bioengineering (2024)
-
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression
Cancer and Metastasis Reviews (2024)
-
Unravelling the mechanotransduction pathways in Alzheimer’s disease
Journal of Biological Engineering (2023)
-
The current status of stimuli-responsive nanotechnologies on orthopedic titanium implant surfaces
Journal of Nanobiotechnology (2023)
-
TMX4-driven LINC complex disassembly and asymmetric autophagy of the nuclear envelope upon acute ER stress
Nature Communications (2023)