Abstract
Altered DNA methylation and associated destabilization of genome integrity and function is a hallmark of cancer. Replicative senescence is a tumour suppressor process that imposes a limit on the proliferative potential of normal cells that all cancer cells must bypass. Here we show by whole-genome single-nucleotide bisulfite sequencing that replicative senescent human cells exhibit widespread DNA hypomethylation and focal hypermethylation. Hypomethylation occurs preferentially at gene-poor, late-replicating, lamin-associated domains and is linked to mislocalization of the maintenance DNA methyltransferase (DNMT1) in cells approaching senescence. Low-level gains of methylation are enriched in CpG islands, including at genes whose methylation and silencing is thought to promote cancer. Gains and losses of methylation in replicative senescence are thus qualitatively similar to those in cancer, and this ‘reprogrammed’ methylation landscape is largely retained when cells bypass senescence. Consequently, the DNA methylome of senescent cells might promote malignancy, if these cells escape the proliferative barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ting, A. H., McGarvey, K. M. & Baylin, S. B. The cancer epigenome–components and functional correlates. Genes Dev. 20, 3215–3231 (2006).
Sproul, D. & Meehan, R. R. Genomic insights into cancer-associated aberrant CpG island hypermethylation. Brief Funct. Genomic. 12, 174–190 (2013).
Hon, G. C. et al. Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome. Res. 22, 246–258 (2012).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Feldser, D. M. & Greider, C. W. Short telomeres limit tumour progression in vivo by inducing senescence. Cancer Cell 11, 461–469 (2007).
Cosme-Blanco, W. et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 8, 497–503 (2007).
Choi, M. R. et al. Genome-scale DNA methylation pattern profiling of human bone marrow mesenchymal stem cells in long-term culture. Exp. Mol. Med. 44, 503–512 (2012).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Zhang, R. et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
O’Sullivan, R. J., Kubicek, S., Schreiber, S. L. & Karlseder, J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 17, 1218–1225 (2010).
Wilson, V. L. & Jones, P. A. DNA methylation decreases in ageing but not in immortal cells. Science 220, 1055–1057 (1983).
Schellenberg, A. et al. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging 3, 873–888 (2011).
Chandra, T. et al. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol. Cell 47, 203–214 (2012).
Sadaie, M. et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 27, 1800–1808 (2013).
Shah, P. P. et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013).
Forsyth, N. R., Evans, A. P., Shay, J. W. & Wright, W. E. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell 2, 235–243 (2003).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumour suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Young, J. I., Sedivy, J. M. & Smith, J. R. Telomerase expression in normal human fibroblasts stabilizes DNA 5-methylcytosine transferase I. J. Biol. Chem. 278, 19904–19908 (2003).
Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462, 315–322 (2009).
Vanderkraats, N. D., Hiken, J. F., Decker, K. F. & Edwards, J. R. Discovering high-resolution patterns of differential DNA methylation that correlate with gene expression changes. Nucleic Acids Res. 41, 6816–6827 (2013).
Leonhardt, H., Page, A. W., Weier, H. U. & Bestor, T. H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71, 865–873 (1992).
Lopatina, N. et al. Differential maintenance and de novo methylating activity by three DNA methyltransferases in ageing and immortalized fibroblasts. J. Cell Biochem. 84, 324–334 (2002).
Suzuki, T., Fujii, M. & Ayusawa, D. Demethylation of classical satellite 2 and 3 DNA with chromosomal instability in senescent human fibroblasts. Exp. Gerontol. 37, 1005–1014 (2002).
Enukashvily, N. I., Donev, R., Waisertreiger, I. S. & Podgornaya, O. I. Human chromosome 1 satellite 3 DNA is decondensed, demethylated and transcribed in senescent cells and in A431 epithelial carcinoma cells. Cytogenet. Genome. Res. 118, 42–54 (2007).
Yamakoshi, K. et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J. Cell Biol. 186, 393–407 (2009).
Young, J. I. & Smith, J. R. DNA methyltransferase inhibition in normal human fibroblasts induces a p21-dependent cell cycle withdrawal. J. Biol. Chem. 276, 19610–19616 (2001).
Aran, D., Toperoff, G., Rosenberg, M. & Hellman, A. Replication timing-related and gene body-specific methylation of active human genes. Hum. Mol. Genet. 20, 670–680 (2011).
Hansen, R. S. et al. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc. Natl Acad. Sci. USA 107, 139–144 (2010).
Moir, R. D., Montag-Lowy, M. & Goldman, R. D. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J. Cell Biol. 125, 1201–1212 (1994).
Peric-Hupkes, D. et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 38, 603–613 (2010).
Yaffe, E. et al. Comparative analysis of DNA replication timing reveals conserved large-scale chromosomal architecture. PLoS Genet. 6, e1001011 (2010).
Berman, B. P. et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat. Genet. 44, 40–46 (2012).
Hansen, K. D. et al. Increased methylation variation in epigenetic domains across cancer types. Nat. Genet. 43, 768–775 (2011).
Ting, D. T. et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science 331, 593–596 (2011).
Toyota, M. et al. CpG island methylator phenotype in colorectal cancer. Proc. Natl Acad. Sci. USA 96, 8681–8686 (1999).
Weisenberger, D. J. et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 38, 787–793 (2006).
Irizarry, R. A et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 41, 178–186 (2009).
De Carvalho, D. D. et al. DNA methylation screening identifies driver epigenetic events of cancer cell survival. Cancer Cell 21, 655–667 (2012).
Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25, 2579–2593 (2011).
Unterberger, A., Andrews, S. D., Weaver, I. C. & Szyf, M. DNA methyltransferase 1 knockdown activates a replication stress checkpoint. Mol. Cell Biol. 26, 7575–7586 (2006).
Suzuki, H. et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signalling in colorectal cancer. Nat. Genet. 36, 417–422 (2004).
Zhang, W. et al. Comparison of global DNA methylation profiles in replicative versus premature senescence. Life Sci. 83, 475–480 (2008).
Casillas, M. A. Jr., Lopatina, N., Andrews, L. G. & Tollefsbol, T. O. Transcriptional control of the DNA methyltransferases is altered in ageing and neoplastically-transformed human fibroblasts. Mol. Cell Biochem. 252, 33–43 (2003).
Eden, A., Gaudet, F., Waghmare, A. & Jaenisch, R. Chromosomal instability and tumours promoted by DNA hypomethylation. Science 300, 455 (2003).
Gaudet, F. et al. Induction of tumours in mice by genomic hypomethylation. Science 300, 489–492 (2003).
Song, J. Z., Stirzaker, C., Harrison, J., Melki, J. R. & Clark, S. J. Hypermethylation trigger of the glutathione-S-transferase gene (GSTP1) in prostate cancer cells. Oncogene 21, 1048–1061 (2002).
Landan, G. et al. Epigenetic polymorphism and the stochastic formation of differentially methylated regions in normal and cancerous tissues. Nat. Genet. 44, 1207–1214 (2012).
O’Hagan, H. M., Mohammad, H. P. & Baylin, S. B. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 4, e1000155 (2008).
Ibrahim, A. E. et al. Sequential DNA methylation changes are associated with DNMT3B overexpression in colorectal neoplastic progression. Gut 60, 499–508 (2011).
Nosho, K. et al. DNMT3B expression might contribute to CpG island methylator phenotype in colorectal cancer. Clin. Cancer Res. 15, 3663–3671 (2009).
Carragher, L. A. et al. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol. Med. 2, 458–471 (2010).
Walsh, C. P., Chaillet, J. R. & Bestor, T. H. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 20, 116–117 (1998).
O’Neill, R. J., O’Neill, M. J. & Graves, J. A. Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature 393, 68–72 (1998).
Lee, E. et al. Landscape of somatic retrotransposition in human cancers. Science 337, 967–971 (2012).
De Cecco, M. et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12, 247–256 (2013).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in ageing skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Herbig, U., Ferreira, M., Condel, L., Carey, D. & Sedivy, J. M. Cellular senescence in ageing primates. Science 311, 1257 (2006).
Sedelnikova, O. A. et al. Senescing human cells and ageing mice accumulateDNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170 (2004).
Feinberg, A. P., Ohlsson, R. & Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 7, 21–33 (2006).
Vredeveld, L. C. et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes. Dev. 26, 1055–1069 (2012).
Kennedy, A. L. et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol. Cell 42, 36–49 (2011).
Pchelintsev, N. A. et al. the HIRA histone chaperone complex in the chromatin landscape. Cell Rep. 3, 1012–1019 (2013).
Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806 (2009).
Harlow, E. & Lane, D. Antibodies: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1988).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572 (2011).
Hansen, K. D., Langmead, B. & Irizarry, R. A. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 13, R83 (2012).
Acknowledgements
Thanks to S. Pepper in the CRUK microarray facility and to S. Hansen for assistance with DNA replication timing data. Thanks to Beijing Genome Institute for bisulfite sequencing. Work in the laboratory of P.D.A. was funded by NIA Program Project P01 AG031862 and CRUK Program A10250. S.L.B.’s laboratory was funded by NIA Program Project P01 AG031862. R.R.M.’s laboratory was funded by the MRC and the BBSRC. P.D.A. thanks P. Cairns for critical formative discussions.
Author information
Authors and Affiliations
Contributions
H.A.C. carried out the bulk of the experiments. D.M.N., P.P.S., J.v.T., T.S.R., C.B., M.E.D. and D.S.D. carried out further experiments. T.M. carried out the bulk of the data analysis. N.D.V. and G.D. carried out further data analyses. H.A.C. and T.M. provided substantial and critical intellectual input. R.R.M., J.R.E. and S.L.B. provided further intellectual input. P.D.A., H.A.C. and T.M. conceived the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Confirmation of senescence in IMR-90 cells.
(A) Growth curve of IMR-90 cells grown in 3% O2 shows proliferation ceased at population doubling (PD) 88. Scale bar = 30 μm. (B) Senescence-Associated β-galactosidase staining in proliferating (prolif), PD28 and senescent (sen), PD88. (C) Quantitation of senescence-associated β-galactosidase (SA β-gal) positive cells. (D) Immunofluorescence of proliferating and senescent cells with a marker of cell proliferation, cyclin A and 4’, 6-diamidino-2-phenylindole (DAPI). Scale bar = 5 μm. (E) Quantitation of cyclin A positive cells. (F) Staining of proliferating and senescent cells with DAPI shows senescent cells displaying senescence associated heterochromatin foci (SAHF). Scale bar = 5 μm. (G) Quantitation of SAHF positive cells. (H) Immunofluorescence of PML and HIRA in proliferating and senescent cells shows co-localization of these 2 proteins, a known marker of senescence in the senescent population. (I) Quantitation of cells with HIRA and PML co-localization. In panels (C), (E), (G) and (I), data was obtained from at least 100 cells scored from a single sample, representative of at least 10 independent samples.
Supplementary Figure 2 Altered gene expression in senescence.
(A) Heatmap showing hierarchical clustering of gene expression in proliferating and senescent cells. Significant changed probes of fold change > = 1.5 and BH-fdr(tt) < = 0.05. (B) Gene set enrichment analysis of downregulated genes with normalized enrichment score (NES) and family wise-error rate (FWER) p-value (C) Gene set enrichment plot of cell cycle process in senescence, top part shows the enrichment value for each gene in this class and the bottom part, the ranked list metric of these genes. (D) Heatmap showing hierarchical clustering of expression of genes in gene set “inflammatory response” (http://www.broadinstitute.org/gsea/msigdb/cards/INFLAMMATORY{_}RESPONSE.html) in proliferating and senescent cells. Significant changed probes of fold change > = 1.5 and BH-fdr(tt) < = 0.05.
Supplementary Figure 3 Concordance of replicates and methylation changes across all chromosomes.
(A) Overlayed percentage methylated basecall plots of proliferating (blue) and senescent (orange) for each replicate pair. Chromosome 1 (chr 1) is shown as a representative region. (B) Difference p plots of all chromosomes (chr).
Supplementary Figure 4 Methylation changes relative to gene expression.
(A)–(C) Relative level of gene expression in proliferating cells against the difference in methylation between proliferating and senescent cells (Sen-Prolif). Methylation was scored at promoters, gene bodies and promoters containing CpG islands defined in UCSC, as indicated. (D)–(F) Ln fold change of gene expression between proliferating and senescent cells (positive values, increased expression in senescence; negative values, decreased expression in senescence) against the difference in methylation between proliferating and senescent cells (Sen-Prolif). Methylation was scored at promoters, gene bodies and promoters containing CpG islands, as indicated. (G) Same analysis as in F, but only for genes expressed above the median level of expression in proliferating cells.
Supplementary Figure 5 Promoters of repressed cell cycle genes are methylated in senescence.
Plots of differential methylation versus position up and downstream of TSS (−5kb to +5kb) for selected genes. Y-axis is a differential methylation score that ranges from −1 to 1, denoting complete hypomethylation and hypermethylation, respectively. The full list of genes is in Supplementary Dataset 3. In each plot, the vertical line marks the TSS. Fold change (log2) gene expression of each gene is indicated in green. For each gene, data from all 3 replicates is shown.
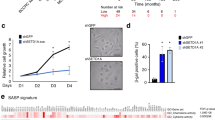
Supplementary Figure 6 Knock down of DNMT1 triggers cell senescence, expression of satellite 2 RNA and senescence-associated chromatin changes; and overlap of hypomethylated DMRs in senescence and cancer.
(A) Proliferating IMR90 fibroblasts were infected with control lentivirus (EV) or lentivirus encoding independent shRNAs to DNMT1 (shDNMT1-a or shDNMT1-b), selected in puromycin and western blotted to detect DNMT1. (B) Cells from (A) were passaged until proliferation arrest. (C–G) After proliferation arrest, cells from (A) were scored by immunofluorescence for expression of cyclin A (C), expression of SA β-gal. (Scale bar = 30 μM), (D), expression of satellite 2 RNA (E), Senescence Associated Heterochromatin Foci (F) and localization of histone chaperone HIRA to PML bodies (G). For (C), (F) and (G) n = 1, but results shown with 2 independent shRNAs and similar results previously reported by others (see main text). (H) Series of graphs assessing percent overlap in total bp over whole genome of indicated features (observed) compared to overlaps calculated for random. Asterisks indicate statistical significance and a p-value of ≪0.001. Hypomethylated cancer (hypo cDMR) and senescence DMRs (hypo sDMR) greater than or equal to 100Kb, greater than or equal to 250Kb, greater than or equal to 500Kb, greater than or equal to 1Mb, greater than or equal to 2Mb, as indicated.
Supplementary Figure 7 Increased methylation of CpG islands in senescence.
Methylation of indicated CpG islands in senescence. Plot of percent methylated basecalls in proliferating (orange) and senescent cells (blue), from whole genome bisulfite sequencing data of 3 replicates of proliferating cells and 3 replicates of senescent cells. The orange and blue lines show the smoothed average percent methylated basecalls at corresponding CpGs. Individual CpGs are indicated by black ticks along the x-axis. The UCSC genes (blue bar) and CpG islands (green bar) are also shown. The transcription start sites (TSS) are indicated by vertical black arrows. Gene, chromosome and bp of CpG island are indicated top left.
Supplementary Figure 8 SV40-infected “bypass” cells proliferate and uncropped versions of Figures.
(A) Bypass cells (SV40) exhibit a low frequency of SA β-gal (<1%). (B) A large proportion of bypass cells (SV40) incorporate a EdU (DNA synthesis) after a 24hr pulse. (C) Quantitation of results from (B), compared to uninfected PD 22 proliferating cells. Error bars indicate standard deviation. Source data for panel (C) can be found in Supplemental Table 22. (D) Uncropped Figure 3c. (E) Uncropped Supplementary Figure 8a. For (D) and (E), see main figures for loading controls. Scale bar in (A) and (B) = 10 μM.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4428 kb)
Supplementary Table 1
Supplementary Information (XLSX 9 kb)
Supplementary Table 2
Supplementary Information (XLSX 8 kb)
Supplementary Table 3
Supplementary Information (XLSX 8 kb)
Supplementary Table 4
Supplementary Information (XLSX 8 kb)
Supplementary Table 5
Supplementary Information (XLSX 8 kb)
Supplementary Table 6
Supplementary Information (XLSX 8 kb)
Supplementary Table 7
Supplementary Information (XLSX 8 kb)
Supplementary Table 8
Supplementary Information (XLSX 7 kb)
Supplementary Table 9
Supplementary Information (XLSX 28 kb)
Supplementary Table 10
Supplementary Information (XLSX 125 kb)
Supplementary Table 11
Supplementary Information (XLSX 20 kb)
Supplementary Table 12
Supplementary Information (XLSX 107 kb)
Supplementary Table 13
Supplementary Information (XLSX 9 kb)
Supplementary Table 14
Supplementary Information (XLSX 9 kb)
Supplementary Table 15
Supplementary Information (XLSX 9 kb)
Supplementary Table 16
Supplementary Information (XLSX 9 kb)
Supplementary Table 17
Supplementary Information (XLSX 11 kb)
Supplementary Table 18
Supplementary Information (XLSX 9 kb)
Supplementary Table 19
Supplementary Information (XLSX 9 kb)
Supplementary Table 20
Supplementary Information (XLSX 9 kb)
Supplementary Table 21
Supplementary Information (XLSX 9 kb)
Supplementary Table 22
Supplementary Information (XLSX 56 kb)
Supplementary Table 23
Supplementary Information (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Cruickshanks, H., McBryan, T., Nelson, D. et al. Senescent cells harbour features of the cancer epigenome. Nat Cell Biol 15, 1495–1506 (2013). https://doi.org/10.1038/ncb2879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2879
This article is cited by
-
Yearning for machine learning: applications for the classification and characterisation of senescence
Cell and Tissue Research (2023)
-
A novel nonparametric computational strategy for identifying differential methylation regions
BMC Bioinformatics (2022)
-
Cell division drives DNA methylation loss in late-replicating domains in primary human cells
Nature Communications (2022)
-
More than causing (epi)genomic instability: emerging physiological implications of transposable element modulation
Journal of Biomedical Science (2021)
-
The role of retrotransposable elements in ageing and age-associated diseases
Nature (2021)