Abstract
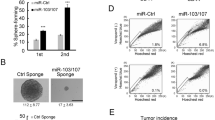
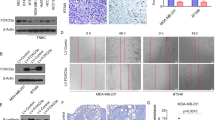
The tumour stroma is an active participant during cancer progression. Stromal cells promote tumour progression and metastasis through multiple mechanisms including enhancing tumour invasiveness and angiogenesis, and suppressing immune surveillance. We report here that miR-126/miR-126*, a microRNA pair derived from a single precursor, independently suppress the sequential recruitment of mesenchymal stem cells and inflammatory monocytes into the tumour stroma to inhibit lung metastasis by breast tumour cells in a mouse xenograft model. miR-126/miR-126* directly inhibit stromal cell-derived factor-1 alpha (SDF-1α) expression, and indirectly suppress the expression of chemokine (C–C motif) ligand 2 (Ccl2) by cancer cells in an SDF-1α-dependent manner. miR-126/miR-126* expression is downregulated in cancer cells by promoter methylation of their host gene Egfl7. These findings determine how this microRNA pair alters the composition of the primary tumour microenvironment to favour breast cancer metastasis, and demonstrate a correlation between miR-126/126* downregulation and poor metastasis-free survival of breast cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005).
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Valastyan, S. & Weinberg, R. A. Tumour metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumour microenvironment. Cancer Cell 21, 309–322 (2012).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumour growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Li, H. J., Reinhardt, F., Herschman, H. R. & Weinberg, R. A. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2, 840–855 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Nicoloso, M. S., Spizzo, R., Shimizu, M., Rossi, S. & Calin, G. A. MicroRNAs–the micro steering wheel of tumour metastases. Nat. Rev. Cancer 9, 293–302 (2009).
Kasinski, A. L. & Slack, F. J. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 11, 849–864 (2011).
Volinia, S. et al. A microRNA expression signature of human solid tumours defines cancer gene targets. Proc. Natl Acad. Sci. USA 103, 2257–2261 (2006).
Ma, L., Teruya-Feldstein, J. & Weinberg, R. A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688 (2007).
Tavazoie, S. F. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147-U143 (2008).
Png, K. J., Halberg, N., Yoshida, M. & Tavazoie, S. F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481, 190–194 (2012).
Crawford, M. et al. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem. Biophys. Res. Commun. 373, 607–612 (2008).
Dong, M. et al. The type III TGF-beta receptor suppresses breast cancer progression. J. Clin. Invest. 117, 206–217 (2007).
Yang, P. et al. TGF-beta-miR-34a-CCL22 signaling-induced treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 22, 291–303 (2012).
Miranda, K. C. et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell 126, 1203–1217 (2006).
Betel, D., Wilson, M., Gabow, A., Marks, D. S. & Sander, C. The microRNA.org resource: targets and expression. Nucl. Acids Res. 36, D149–D153 (2008).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 (2009).
Stenvang, J., Petri, A., Lindow, M., Obad, S. & Kauppinen, S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 3, 1 (2012).
Kryczek, I. et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 65, 465–472 (2005).
Aiuti, A., Webb, I. J., Bleul, C., Springer, T. & GutierrezRamos, J. C. The chemokine SDF-1 is a chemoattractant for human CD34(+) hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34(+) progenitors to peripheral blood. J. Exp. Med. 185, 111–120 (1997).
Ponte, A. L. et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25, 1737–1745 (2007).
Kitaori, T. et al. Stromal cell-derived factor 1/CXCR4 signalling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 60, 813–823 (2009).
Son, B. R. et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24, 1254–1264 (2006).
Soleimani, M. & Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 4, 102–106 (2009).
Broxmeyer, H. E. et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 (2005).
Fox, J. M., Chamberlain, G., Ashton, B. A. & Middleton, J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br. J. Haematol. 137, 491–502 (2007).
Meirelles Lda, S. & Nardi, N. B. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br. J. Haematol. 123, 702–711 (2003).
Baddoo, M. et al. Characterization of mesenchymal stem cells isolated frommurine bone marrow by negative selection. J. Cell Biochem. 89, 1235–1249 (2003).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Aslakson, C. J. & Miller, F. R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumour. Cancer Res. 52, 1399–1405 (1992).
Saito, Y. et al. Epigenetic therapy upregulates the tumour suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem. Biophys. Res. Commun. 379, 726–731 (2009).
Marchesi, F. et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumour cells expressing functional CXCR4. Cancer Res. 64, 8420–8427 (2004).
Miao, Z. et al. CXCR7 (RDC1) promotes breast and lung tumour growth in vivo and is expressed on tumour-associated vasculature. Proc. Natl Acad. Sci. USA 104, 15735–15740 (2007).
Kedde, M. et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007).
Kedde, M. et al. A Pumilio-induced RNA structure switch in p27-3′ UTRcontrols miR-221 and miR-222 accessibility. Nat. Cell Biol. 12, 1014–1020 (2010).
Ebert, M. S., Neilson, J. R. & Sharp, P. A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 4, 721–726 (2007).
Ma, C. et al. Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev. 22, 308–321 (2008).
Rubin, J. B. et al. A small-molecule antagonist of CXCR4 inhibits intracranialgrowth of primary brain tumours. Proc. Natl Acad. Sci. USA 100, 13513–13518 (2003).
Chi, S. W., Zang, J. B., Mele, A. & Darnell, R. B. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460, 479–486 (2009).
Ule, J. et al. CLIP identifies Nova-regulated RNA networks in the brain. Science 302, 1212–1215 (2003).
Li, L. C. & Dahiya, R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002).
Acknowledgements
The authors thank R. A. Weinberg, L. M. Wakefield, C. Counter andA. M. Pendergast for providing reagents. We also thank G. J. Markowitz for thoughtful comments on the manuscript. This work was supported by the NIH grant CA151541 to X-F.W., the S. G. Komen For The Cure Foundation grant KG101633 to X-F.W. (PI) and X.X. (Fellow), the Research Scholar Grant RSG-10-157-01-LIB from the American Cancer Society to Q-J.L. and Ministry of Science and Technology Key Program of China 2012ZX10002009-017, National Basic Research Program of China 2010CB912102 to D.X.
Author information
Authors and Affiliations
Contributions
Y.Z., P.Y. and X.W. designed the research; Y.Z. and P.Y. performed most experiments and analysed the results; T.S., X.X., C.L. and M.C. provided further technical assistance; D-J.L. and Y.R. provided technical assistance in luciferase reporter assay and MSCs isolation; T.I., L.M., D.A. and X.L. provided clinical samples and associated analyses; Y.Z. and X-F.W. wrote the manuscript; P.Y., D.X. and Q-J.L. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2344 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Yang, P., Sun, T. et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol 15, 284–294 (2013). https://doi.org/10.1038/ncb2690
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2690
This article is cited by
-
Macrophages and angiogenesis in human lymphomas
Clinical and Experimental Medicine (2024)
-
Adipose tissue macrophages: implications for obesity-associated cancer
Military Medical Research (2023)
-
The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis
Cellular & Molecular Immunology (2023)
-
Role of senescent tumor cells in building a cytokine shield in the tumor microenvironment: mathematical modeling
Journal of Mathematical Biology (2023)
-
One locus, several functional RNAs—emerging roles of the mechanisms responsible for the sequence variability of microRNAs
Biologia Futura (2023)