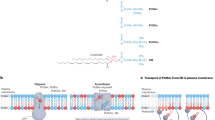
Abstract
Lipid asymmetry at the plasma membrane is essential for such processes as cell polarity, cytokinesis and phagocytosis1,2,3. Here we find that a lipid flippase complex, composed of Lem3, Dnf1 or Dnf2 (ref. 4), has a role in the dynamic recycling of the Cdc42 GTPase, a key regulator of cell polarity5, in yeast. By using quantitative microscopy methods, we show that the flippase complex is required for fast dissociation of Cdc42 from the polar cortex by the guanine nucleotide dissociation inhibitor. A loss of flippase activity, or pharmacological blockage of the inward flipping of phosphatidylethanolamine, a phospholipid with a neutral head group, disrupts Cdc42 polarity maintained by guanine nucleotide dissociation inhibitor-mediated recycling. Phosphatidylethanolamine flipping may reduce the charge interaction between a Cdc42 carboxy-terminal cationic region with the plasma membrane inner leaflet, enriched for the negatively charged lipid phosphatidylserine. Using a reconstituted system with supported lipid bilayers, we show that the relative composition of phosphatidylethanolamine versus phosphatidylserine directly modulates Cdc42 extraction from the membrane by guanine nucleotide dissociation inhibitor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Iwamoto, K. et al. Local exposure of phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity. Genes Cells 9, 891–903 (2004).
Yeung, T. et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 (2008).
Emoto, K. & Umeda, M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell Biol. 149, 1215–1224 (2000).
Pomorski, T. & Menon, A. K. Lipid flippases and their biological functions. Cell Mol. Life Sci. 63, 2908–2921 (2006).
Etienne-Manneville, S. Cdc42—the centre of polarity. J. Cell Sci. 117, 1291–1300 (2004).
Slaughter, B. D., Smith, S. E. & Li, R. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harb. Perspect Biol. 1, a003384 (2009).
Wedlich-Soldner, R., Wai, S. C., Schmidt, T. & Li, R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166, 889–900 (2004).
Marco, E., Wedlich-Soldner, R., Li, R., Altschuler, S. J. & Wu, L. F. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell 129, 411–422 (2007).
Slaughter, B. D., Das, A., Schwartz, J. W., Rubinstein, B. & Li, R. Dual modes of Cdc42 recycling fine-tune polarized morphogenesis. Dev. Cell 17, 823–835 (2009).
Masuda, T. et al. Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem. 269, 19713–19718 (1994).
DerMardirossian, C. & Bokoch, G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Johnson, J. L., Erickson, J. W. & Cerione, R. A. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J. Biol. Chem. 284, 23860–23871 (2009).
Richman, T. J. et al. Analysis of cell-cycle specific localization of the Rdi1p RhoGDI and the structural determinants required for Cdc42p membrane localization and clustering at sites of polarized growth. Curr. Genet. 45, 339–349 (2004).
Winzeler, E. A. et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 (1999).
Pomorski, T. et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 14, 1240–1254 (2003).
Kato, U. et al. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J. Biol. Chem. 277, 37855–37862 (2002).
Saito, K. et al. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev. Cell 13, 743–751 (2007).
Slaughter, B. D. & Li, R. Toward quantitative ‘in vivo biochemistry’ with fluorescence fluctuation spectroscopy. Mol. Biol. Cell 21, 4306–4311 (2010).
Gibson, R. M. & Wilson-Delfosse, A. L. RhoGDI-binding-defective mutant of Cdc42Hs targets to membranes and activates filopodia formation but does not cycle with the cytosol of mammalian cells. Biochem. J. 359, 285–294 (2001).
Grossmann, G. et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell Biol. 183, 1075–1088 (2008).
Ayscough, K. R. et al. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399–416 (1997).
Aoki, Y., Uenaka, T., Aoki, J., Umeda, M. & Inoue, K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J. Biochem. 116, 291–297 (1994).
Stevens, H. C., Malone, L. & Nichols, J. W. The putative aminophospholipid translocases, DNF1 and DNF2, are not required for 7-nitrobenz-2-oxa-1,3-diazol-4-yl-phosphatidylserine flip across the plasma membrane of Saccharomyces cerevisiae. J. Biol. Chem. 283, 35060–35069 (2008).
Stefan, C. J., Audhya, A. & Emr, S. D. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13, 542–557 (2002).
Forget, M. A., Desrosiers, R. R., Gingras, D. & Beliveau, R. Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 361, 243–254 (2002).
Griffin, B. A., Adams, S. R. & Tsien, R. Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Fairn, G. D., Hermansson, M., Somerharju, P. & Grinstein, S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat. Cell Biol. 13, 1424–1430 (2011).
Caviston, J. P., Longtine, M., Pringle, J. R. & Bi, E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051–4066 (2003).
Slaughter, B. D., Schwartz, J. W. & Li, R. Mapping dynamic protein interactions in MAP kinase signaling using live-cell fluorescence fluctuation spectroscopy and imaging. Proc. Natl Acad. Sci. USA 104, 20320–20325 (2007).
Kim, S. A., Heinze, K. G. & Schwille, P. Fluorescence correlation spectroscopy in living cells. Nat. Methods 4, 963–973 (2007).
Haupts, U., Maiti, S., Schwille, P. & Webb, W. W. Dynamics of fluorescence fluctuations in green fluorescent protein observed by fluorescence correlation spectroscopy. Proc. Natl Acad. Sci. USA 95, 13573–13578 (1998).
Bevington, P. & Robinson, D. K. Data Reduction and Error Analysis for the Physical Sciences 3rd edn 194–218 (McGraw-Hill, 2003).
Thompson, N. L. Fluorescence Correlation Spectroscopy. Topics in Fluorescence Spectroscopy 337–378 (Plenum Press, 1991).
Coles, B. A. & Compton, R. G. Photoelectrochemical ESR. Part I. Experimental. J. Electroanal. Chem. Interfacial Electrochem. 144, 87–98 (1983).
Daly, P. J., Page, D. J. & Compton, R. G. Mercury-plated rotating ring-disk electrode. Anal. Chem. 55, 1191–1192 (1983).
Hess, S. T. & Webb, W. W. Focal volume optics and experimental artifacts in confocal fluorescence correlation spectroscopy. Biophys. J. 83, 2300–2317 (2002).
Martin, B. R., Giepmans, B. N., Adams, S. R. & Tsien, R. Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23, 1308–1314 (2005).
Goud, B., Salminen, A., Walworth, N. C. & Novick, P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53, 753–768 (1988).
Lee, K., Gallop, J. L., Rambani, K. & Kirschner, M. W. Self-assembly of filopodia-like structures on supported lipid bilayers. Science 329, 1341–1345 (2010).
Poste, G., Papahadjopoulos, D. & Vail, W. J. In Methods in Cell Biology 34–72 (Academic Press, 1976).
Acknowledgements
The authors thank W. Wiegraebe (Stowers Institute for Medical Research) for advice on imaging, K. Lee (Harvard Medical School) for advice on supported lipid bilayer preparation and N. Pavelka for assistance in genome screening data analysis. This study was done to fulfil, in part, requirements for A.D.’s PhD thesis as a student registered with the Open University. This work was supported by NIH grant RO1-GM057063 to R.L.
Author information
Authors and Affiliations
Contributions
A.D. and R.L. designed the experiments; A.D. carried out all experiments and prepared the manuscript, with help from B.D.S. and J.R.U.; R.A. and W.D.B. assisted in the whole genome screening; B.R. assisted in data analysis; R.L. conceived and supervised the project and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 444 kb)
Supplementary Table 1
Supplementary Information (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Das, A., Slaughter, B., Unruh, J. et al. Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nat Cell Biol 14, 304–310 (2012). https://doi.org/10.1038/ncb2444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2444
This article is cited by
-
P4-ATPase subunit Cdc50 plays a role in yeast budding and cell wall integrity in Candida glabrata
BMC Microbiology (2023)
-
Redundancy and the role of protein copy numbers in the cell polarization machinery of budding yeast
Nature Communications (2023)
-
Lipid flippases in polarized growth
Current Genetics (2021)
-
Phospholipid flippase ATP11C is endocytosed and downregulated following Ca2+-mediated protein kinase C activation
Nature Communications (2017)
-
P4-ATPases: lipid flippases in cell membranes
Pflügers Archiv - European Journal of Physiology (2014)