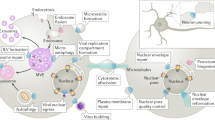
Abstract
Clathrin-mediated endocytosis (CME) is the major pathway for internalization of membrane proteins from the cell surface. Half a century of studies have uncovered tremendous insights into how a clathrin-coated vesicle is formed. More recently, the advent of live-cell imaging has provided a dynamic view of this process. As CME is highly conserved from yeast to humans, budding yeast provides an evolutionary template for this process and has been a valuable system for dissecting the underlying molecular mechanisms. In this review we trace the formation of a clathrin-coated vesicle from initiation to uncoating, focusing on key findings from the yeast system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Roth, T. F. & Porter, K. R. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. J. Cell Biol. 20, 313–332 (1964).
Crowther, R. A., Finch, J. T. & Pearse, B. M. On the structure of coated vesicles. J. Mol. Biol. 103, 785–798 (1976).
Pearse, B. M. Coated vesicles from pig brain: purification and biochemical characterization. J. Mol. Biol. 97, 93–98 (1975).
Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C. & Wakeham, D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568 (2001).
Raths, S., Rohrer, J., Crausaz, F. & Riezman, H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 120, 55–65 (1993).
Wendland, B., McCaffery, J. M., Xiao, Q. & Emr, S. D. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J. Cell Biol. 135, 1485–1500 (1996).
Munn, A. L., Stevenson, B. J., Geli, M. I. & Riezman, H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 6, 1721–1742 (1995).
Kubler, E. & Riezman, H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 12, 2855–2862 (1993).
Kaksonen, M., Sun, Y. & Drubin, D. G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475–487 (2003).
Kaksonen, M., Toret, C. P. & Drubin, D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305–320 (2005).
Newpher, T. M., Smith, R. P., Lemmon, V. & Lemmon, S. K. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev. Cell 9, 87–98 (2005).
Sirotkin, V., Berro, J., Macmillan, K., Zhao, L. & Pollard, T. D. Quantitative analysis of the mechanism of endocytic actin patch assembly and disassembly in fission yeast. Mol. Biol. Cell 21, 2894–2904 (2010).
Idrissi, F. Z. et al. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J. Cell Biol. 180, 1219–1232 (2008).
Stefan, C. J., Audhya, A. & Emr, S. D. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4, 5)-bisphosphate. Mol. Biol. Cell 13, 542–557 (2002).
Antonescu, C. N., Aguet, F., Danuser, G. & Schmid, S. L. Phosphatidylinositol-(4, 5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol. Biol. Cell 22, 2588–2600 (2011).
Boettner, D. R. et al. The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr. Biol. 19, 1979–1987 (2009).
Reider, A. et al. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 28, 3103–3116 (2009).
Stimpson, H. E., Toret, C. P., Cheng, A. T., Pauly, B. S. & Drubin, D. G. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol. Biol. Cell 20, 4640–4651 (2009).
Toshima, J. Y. et al. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc. Natl Acad. Sci. USA 103, 5793–5798 (2006).
Newpher, T. M. & Lemmon, S. K. Clathrin is important for normal actin dynamics and progression of Sla2p-containing patches during endocytosis in yeast. Traffic 7, 574–588 (2006).
Toret, C. P., Lee, L., Sekiya-Kawasaki, M. & Drubin, D. G. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic 9, 848–859 (2008).
Payne, G. S., Baker, D., van Tuinen, E. & Schekman, R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J. Cell Biol. 106, 1453–1461 (1988).
Huang, K. M. et al. Novel functions of clathrin light chains: clathrin heavy chain trimerization is defective in light chain-deficient yeast. J. Cell Sci. 110, 899–910 (1997).
Newpher, T. M., Idrissi, F. Z., Geli, M. I. & Lemmon, S. K. Novel function of clathrin light chain in promoting endocytic vesicle formation. Mol. Biol. Cell 17, 4343–4352 (2006).
Gagny, B. et al. A novel EH domain protein of Saccharomyces cerevisiae, Ede1p, involved in endocytosis. J. Cell Sci. 113, 3309–3319 (2000).
Henne, W. M. et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328, 1281–1284 (2010).
Dell'Angelica, E. C. Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol. 11, 315–318 (2001).
Collette, J. R. et al. Clathrin functions in the absence of the terminal domain binding site for adaptor-associated clathrin-box motifs. Mol. Biol. Cell 20, 3401–3413 (2009).
Kang, D. S. et al. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J. Biol. Chem. 284, 29860–29872 (2009).
Willox, A. K. & Royle, S. J. Functional analysis of interaction sites on the N-terminal domain of clathrin heavy chain. Traffic 13, 70–81 (2011).
Carroll, S. Y. et al. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev. Cell 17, 552–560 (2009).
Huang, K. M., D'Hondt, K., Riezman, H. & Lemmon, S. K. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18, 3897–3908 (1999).
Yeung, B. G., Phan, H. L. & Payne, G. S. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 10, 3643–3659 (1999).
Burston, H. E. et al. Regulators of yeast endocytosis identified by systematic quantitative analysis. J. Cell Biol. 185, 1097–1110 (2009).
Maldonado-Baez, L. et al. Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol. Biol. Cell 19, 2936–2948 (2008).
Howard, J. P., Hutton, J. L., Olson, J. M. & Payne, G. S. Sla1p serves as the targeting signal recognition factor for NPFX(1, 2)D-mediated endocytosis. J. Cell Biol. 157, 315–326 (2002).
Piao, H. L., Machado, I. M. & Payne, G. S. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol. Biol. Cell 18, 57–65 (2007).
Di Pietro, S. M., Cascio, D., Feliciano, D., Bowie, J. U. & Payne, G. S. Regulation of clathrin adaptor function in endocytosis: novel role for the SAM domain. EMBO J. 29, 1033–1044 (2010).
Shih, S. C. et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4, 389–393 (2002).
Dores, M. R., Schnell, J. D., Maldonado-Baez, L., Wendland, B. & Hicke, L. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic 11, 151–160 (2010).
Stamenova, S. D. et al. Ubiquitin binds to and regulates a subset of SH3 domains. Mol. Cell 25, 273–284 (2007).
Moseley, J. B. & Goode, B. L. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70, 605–645 (2006).
Jonsdottir, G. A. & Li, R. Dynamics of yeast Myosin I: evidence for a possible role in scission of endocytic vesicles. Curr. Biol. 14, 1604–1609 (2004).
Sun, Y., Martin, A. C. & Drubin, D. G. Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev. Cell 11, 33–46 (2006).
Sirotkin, V., Beltzner, C. C., Marchand, J. B. & Pollard, T. D. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J. Cell Biol. 170, 637–648 (2005).
Toshima, J. et al. Negative regulation of yeast Eps15-like Arp2/3 complex activator, Pan1p, by the Hip1R-related protein, Sla2p, during endocytosis. Mol. Biol. Cell 18, 658–668 (2007).
Rodal, A. A., Manning, A. L., Goode, B. L. & Drubin, D. G. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 13, 1000–1008 (2003).
Soulard, A. et al. The WASP/Las17p-interacting protein Bzz1p functions with Myo5p in an early stage of endocytosis. Protoplasma 226, 89–101 (2005).
Grotsch, H. et al. Calmodulin dissociation regulates Myo5 recruitment and function at endocytic sites. EMBO J. 29, 2899–2914 (2010).
Galletta, B. J., Chuang, D. Y. & Cooper, J. A. Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 6, e1 (2008).
Merrifield, C. J., Qualmann, B., Kessels, M. M. & Almers, W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 83, 13–18 (2004).
Merrifield, C. J., Perrais, D. & Zenisek, D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593–606 (2005).
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Dharmalingam, E. et al. F-BAR proteins of the syndapin family shape the plasma membrane and are crucial for neuromorphogenesis. J. Neurosci. 29, 13315–13327 (2009).
Kessels, M. M. & Qualmann, B. Syndapin oligomers interconnect the machineries for endocytic vesicle formation and actin polymerization. J. Biol. Chem. 281, 13285–13299 (2006).
Koch, D. et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. http://dx.doi.org/10.1038/emboj.2011.339 (2011).
Rocca, D. L., Martin, S., Jenkins, E. L. & Hanley, J. G. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat. Cell Biol. 10, 259–271 (2008).
Engqvist-Goldstein, A. E., Kessels, M. M., Chopra, V. S., Hayden, M. R. & Drubin, D. G. An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J. Cell Biol. 147, 1503–1518 (1999).
Wesp, A. et al. End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 2291–2306 (1997).
Yang, S., Cope, M. J. & Drubin, D. G. Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol. Biol. Cell 10, 2265–2283 (1999).
Engqvist-Goldstein, A. E. et al. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol. Biol. Cell 15, 1666–1679 (2004).
Sun, Y., Carroll, S., Kaksonen, M., Toshima, J. Y. & Drubin, D. G. PtdIns(4, 5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J. Cell Biol. 177, 355–367 (2007).
Sun, Y., Kaksonen, M., Madden, D. T., Schekman, R. & Drubin, D. G. Interaction of Sla2p's ANTH domain with PtdIns(4, 5)P2 is important for actin-dependent endocytic internalization. Mol. Biol. Cell 16, 717–730 (2005).
Gourlay, C. W. et al. An interaction between Sla1p and Sla2p plays a role in regulating actin dynamics and endocytosis in budding yeast. J. Cell Sci. 116, 2551–2564 (2003).
Brett, T. J., Legendre-Guillemin, V., McPherson, P. S. & Fremont, D. H. Structural definition of the F-actin-binding THATCH domain from HIP1R. Nat. Struct. Mol. Biol. 13, 121–130 (2006).
McCann, R. O. & Craig, S. W. The I/LWEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc. Natl Acad. Sci. USA 94, 5679–5684 (1997).
Wilbur, J. D. et al. Actin binding by Hip1 (huntingtin-interacting protein 1) and Hip1R (Hip1-related protein) is regulated by clathrin light chain. J. Biol. Chem. 283, 32870–32879 (2008).
Engqvist-Goldstein, A. E. et al. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 154, 1209–1223 (2001).
Boettner, D. R., Friesen, H., Andrews, B. & Lemmon, S. K. Clathrin light chain directs endocytosis by influencing the binding of the yeast Hip1R homologue, Sla2, to F-actin. Mol. Biol. Cell 22, 3699–3714 (2011).
Baggett, J. J., D'Aquino, K. E. & Wendland, B. The Sla2p talin domain plays a role in endocytosis in Saccharomyces cerevisiae. Genetics 165, 1661–1674 (2003).
Smaczynska-de, R., II. et al. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J. Cell Sci. 123, 3496–3506 (2010).
Youn, J. Y. et al. Dissecting BAR domain function in the yeast Amphiphysins Rvs161 and Rvs167 during endocytosis. Mol. Biol. Cell 21, 3054–3069 (2010).
Sever, S., Damke, H. & Schmid, S. L. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150, 1137–1148 (2000).
Nothwehr, S. F., Conibear, E. & Stevens, T. H. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol. 129, 35–46 (1995).
Nannapaneni, S. et al. The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. Eur. J. Cell Biol. 89, 499–508 (2010).
Liu, J., Sun, Y., Drubin, D. G. & Oster, G. F. The mechanochemistry of endocytosis. PLoS Biol. 7, e1000204 (2009).
Liu, J., Kaksonen, M., Drubin, D. G. & Oster, G. Endocytic vesicle scission by lipid phase boundary forces. Proc. Natl Acad. Sci. USA 103, 10277–10282 (2006).
Stefan, C. J., Padilla, S. M., Audhya, A. & Emr, S. D. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol. Cell Biol. 25, 2910–2923 (2005).
Singer-Kruger, B., Nemoto, Y., Daniell, L., Ferro-Novick, S. & De Camilli, P. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J. Cell Sci. 111, 3347–3356 (1998).
Arasada, R. & Pollard, T. D. Distinct roles for F-BAR proteins Cdc15p and Bzz1p in actin polymerization at sites of endocytosis in fission yeast. Curr. Biol. 21, 1450–1459 (2011).
Kishimoto, T., Sun, Y., Buser, C., Liu, J., Michelot, A., Drubin, D. G. Determinants of endocytic membrane geometry, stability, and scission. Proc. Natl Acad. Sci. USA 44, E979–E988 (2011).
Ferguson, S. M. et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell 17, 811–822 (2009).
Wu, M. et al. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat. Cell Biol. 12, 902–908 (2010).
Yamada, H. et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J. Biol. Chem. 284, 34244–34256 (2009).
Cope, M. J., Yang, S., Shang, C. & Drubin, D. G. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol. 144, 1203–1218 (1999).
Smythe, E. & Ayscough, K. R. The Ark1/Prk1 family of protein kinases. Regulators of endocytosis and the actin skeleton. EMBO Rep. 4, 246–251 (2003).
Zeng, G., Huang, B., Neo, S. P., Wang, J. & Cai, M. Scd5p mediates phosphoregulation of actin and endocytosis by the type 1 phosphatase Glc7p in yeast. Mol. Biol. Cell 18, 4885–4898 (2007).
Jin, M. & Cai, M. A novel function of Arp2p in mediating Prk1p-specific regulation of actin and endocytosis in yeast. Mol. Biol. Cell 19, 297–307 (2008).
Huang, B., Zeng, G., Ng, A. Y. & Cai, M. Identification of novel recognition motifs and regulatory targets for the yeast actin-regulating kinase Prk1p. Mol. Biol. Cell 14, 4871–4884 (2003).
Watson, H. A., Cope, M. J., Groen, A. C., Drubin, D. G. & Wendland, B. In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation. Mol. Biol. Cell 12, 3668–3679 (2001).
Henry, K. R. et al. The actin-regulating kinase Prk1p negatively regulates Scd5p, a suppressor of clathrin deficiency, in actin organization and endocytosis. Curr. Biol. 13, 1564–1569 (2003).
Zeng, G., Yu, X. & Cai, M. Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol. Biol. Cell 12, 3759–3772 (2001).
Zeng, G. & Cai, M. Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J. Cell Biol. 144, 71–82 (1999).
Breitkreutz, A. et al. A global protein kinase and phosphatase interaction network in yeast. Science 328, 1043–1046 (2010).
Sekiya-Kawasaki, M. et al. Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis. J. Cell Biol. 162, 765–772 (2003).
Chang, J. S., Henry, K., Geli, M. I. & Lemmon, S. K. Cortical recruitment and nuclear-cytoplasmic shuttling of Scd5p, a protein phosphatase-1-targeting protein involved in actin organization and endocytosis. Mol. Biol. Cell 17, 251–262 (2006).
Toshima, J., Toshima, J. Y., Martin, A. C. & Drubin, D. G. Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nat. Cell Biol. 7, 246–254 (2005).
Chang, J. S., Henry, K., Wolf, B. L., Geli, M. & Lemmon, S. K. Protein phosphatase-1 binding to scd5p is important for regulation of actin organization and endocytosis in yeast. J. Biol. Chem. 277, 48002–48008 (2002).
Henry, K. R. et al. Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of sla2p in yeast. Mol. Biol. Cell 13, 2607–2625 (2002).
Tonikian, R. et al. Bayesian modeling of the yeast SH3 domain interactome predicts spatiotemporal dynamics of endocytosis proteins. PLoS Biol. 7, e1000218 (2009).
Honing, S. et al. Phosphatidylinositol-(4, 5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell 18, 519–531 (2005).
Jackson, L. P. et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141, 1220–1229 (2010).
Ricotta, D., Conner, S. D., Schmid, S. L., von Figura, K. & Honing, S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol. 156, 791–795 (2002).
Greener, T., Zhao, X., Nojima, H., Eisenberg, E. & Greene, L. E. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J. Biol. Chem. 275, 1365–1370 (2000).
Lee, D. W., Wu, X., Eisenberg, E. & Greene, L. E. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J. Cell Sci. 119, 3502–3512 (2006).
Umeda, A., Meyerholz, A. & Ungewickell, E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 79, 336–342 (2000).
Zhang, C. X. et al. Multiple roles for cyclin G-associated kinase in clathrin-mediated sorting events. Traffic 6, 1103–1113 (2005).
Cousin, M. A., Tan, T. C. & Robinson, P. J. Protein phosphorylation is required for endocytosis in nerve terminals: potential role for the dephosphins dynamin I and synaptojanin, but not AP180 or amphiphysin. J. Neurochem. 76, 105–116 (2001).
Lee, S. Y., Wenk, M. R., Kim, Y., Nairn, A. C. & De Camilli, P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl Acad. Sci. USA 101, 546–551 (2004).
Slepnev, V. I., Ochoa, G. C., Butler, M. H., Grabs, D. & De Camilli, P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281, 821–824 (1998).
Tan, T. C. et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 5, 701–710 (2003).
Okreglak, V. & Drubin, D. G. Cofilin recruitment and function during actin-mediated endocytosis dictated by actin nucleotide state. J. Cell Biol. 178, 1251–1264 (2007).
Lin, M. C., Galletta, B. J., Sept, D. & Cooper, J. A. Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J. Cell Sci. 123, 1329–1342 (2010).
Bobkov, A. A. et al. Cooperative effects of cofilin (ADF) on actin structure suggest allosteric mechanism of cofilin function. J. Mol. Biol. 356, 325–334 (2006).
McGough, A., Pope, B., Chiu, W. & Weeds, A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell. Biol. 138, 771–781 (1997).
Muhlrad, A. et al. Cofilin induced conformational changes in F-actin expose subdomain 2 to proteolysis. J. Mol. Biol. 342, 1559–1567 (2004).
Balcer, H. I. et al. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159–2169 (2003).
Okada, K., Ravi, H., Smith, E. M. & Goode, B. L. Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol. Biol. Cell 17, 2855–2868 (2006).
Bertling, E., Quintero-Monzon, O., Mattila, P. K., Goode, B. L. & Lappalainen, P. Mechanism and biological role of profilin-Srv2/CAP interaction. J. Cell Sci. 120, 1225–1234 (2007).
Quintero-Monzon, O. et al. Reconstitution and dissection of the 600-kDa Srv2/CAP complex: roles for oligomerization and cofilin-actin binding in driving actin turnover. J. Biol. Chem. 284, 10923–10934 (2009).
Chaudhry, F., Little, K., Talarico, L., Quintero-Monzon, O. & Goode, B. L. A central role for the WH2 domain of Srv2/CAP in recharging actin monomers to drive actin turnover in vitro and in vivo. Cytoskeleton (Hoboken) 67, 120–133 (2010).
Gandhi, M. et al. GMF is a cofilin homolog that binds Arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Curr. Biol. 20, 861–867 (2010).
Doyon, J. B. et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat. Cell Biol. 13, 331–337 (2011).
Batchelder, E. M. & Yarar, D. Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol. Biol. Cell 21, 3070–3079 (2010).
Collins, A., Warrington, A., Taylor, K. A. & Svitkina, T. Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr. Biol. 21, 1167–1175 (2011).
Cureton, D. K., Massol, R. H., Saffarian, S., Kirchhausen, T. L. & Whelan, S. P. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5, e1000394 (2009).
Saffarian, S., Cocucci, E. & Kirchhausen, T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 7, e1000191 (2009).
Boulant, S., Kural, C., Zeeh, J. C., Ubelmann, F. & Kirchhausen, T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 13, 1124–1131 (2011).
Liu, A. P., Loerke, D., Schmid, S. L. & Danuser, G. Global and local regulation of clathrin-coated pit dynamics detected on patterned substrates. Biophys. J. 97, 1038–1047 (2009).
Hohmann, S., Krantz, M. & Nordlander, B. Yeast osmoregulation. Methods Enzymol. 428, 29–45 (2007).
Aghamohammadzadeh, S. & Ayscough, K. R. Differential requirements for actin during yeast and mammalian endocytosis. Nat. Cell Biol. 11, 1039–1042 (2009).
Prosser, D. C., Drivas, T. G., Maldonado-Báez, L., Wendland, B. Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J. Cell Biol. 195, 657–671 (2011).
Acknowledgements
We would like to thank J. A. Cooper and B. J. Galletta for insightful discussions, and C. Fahrenholtz for critical comments on the manuscript. This work was supported by grants and fellowships from the National Institutes of Health: F32-GM084677 (DRB), T32-HL07188 (RJC), F32-GM087900 (RJC), and R01-GM055796 (SKL). Finally, we apologize to colleagues whose work we were unable to cite in this brief review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Boettner, D., Chi, R. & Lemmon, S. Lessons from yeast for clathrin-mediated endocytosis. Nat Cell Biol 14, 2–10 (2012). https://doi.org/10.1038/ncb2403
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2403
This article is cited by
-
Characterization of the complex involved in regulating V-ATPase activity of the vacuolar and endosomal membrane
Journal of Bioenergetics and Biomembranes (2017)
-
The microRNA-23b/-27b cluster suppresses prostate cancer metastasis via Huntingtin-interacting protein 1-related
Oncogene (2016)
-
The long life of an endocytic patch that misses AP-2
Current Genetics (2016)
-
Intrinsically disordered proteins drive membrane curvature
Nature Communications (2015)
-
Clathrin light chains are required for the gyrating-clathrin recycling pathway and thereby promote cell migration
Nature Communications (2014)