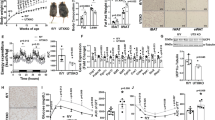
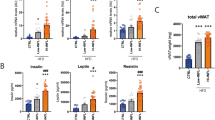
Abstract
Mammals have two principal types of fat. White adipose tissue primarily serves to store extra energy as triglycerides, whereas brown adipose tissue is specialized to burn lipids for heat generation and energy expenditure as a defence against cold and obesity1,2. Recent studies have demonstrated that brown adipocytes arise in vivo from a Myf5-positive, myoblastic progenitor by the action of Prdm16 (PR domain containing 16). Here, we identified a brown-fat-enriched miRNA cluster, MiR-193b–365, as a key regulator of brown fat development. Blocking miR-193b and/or miR-365 in primary brown preadipocytes markedly impaired brown adipocyte adipogenesis by enhancing Runx1t1 (runt-related transcription factor 1; translocated to, 1) expression, whereas myogenic markers were significantly induced. Forced expression of Mir193b and/or Mir365 in C2C12 myoblasts blocked the entire programme of myogenesis, and, in adipogenic conditions, miR-193b induced myoblasts to differentiate into brown adipocytes. Mir193b–365 was upregulated by Prdm16 partially through Pparα. Our results demonstrate that Mir193b–365 serves as an essential regulator for brown fat differentiation, in part by repressing myogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seale, P., Kajimura, S. & Spiegelman, B. M. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 23, 788–797 (2009).
Gesta, S., Tseng, Y. H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. New Engl. J. Med. 360, 1509–1517 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. New Engl. J. Med. 360, 1500–1508 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. New Engl. J. Med. 360, 1518–1525 (2009).
Connolly, E., Morrisey, R. D. & Carnie, J. A. The effect of interscapular brown adipose tissue removal on body-weight and cold response in the mouse. Br. J. Nutr. 47, 653–658 (1982).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993).
Hamann, A., Flier, J. S. & Lowell, B. B. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137, 21–29 (1996).
Feldmann, H. M., Golozoubova, V., Cannon, B. & Nedergaard, J. Ucp1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 (2009).
Kopecky, J., Clarke, G., Enerback, S., Spiegelman, B. & Kozak, L. P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Invest. 96, 2914–2923 (1995).
Kopecky, J. et al. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am. J. Physiol. 270, E776–E786 (1996).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205.
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Kajimura, S. et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature 460, 1154–1158 (2009).
Kawaji, H. et al. CAGE Basic/Analysis Databases: the CAGE resource for comprehensive promoter analysis. Nucleic Acids Res. 34, D632–D636 (2006).
Carninci, P. et al. The transcriptional landscape of the mammalian genome. Science 309, 1559–1563 (2005).
Hogan, S. & Himms-Hagen, J. Abnormal brown adipose tissue in obese (ob/ob) mice: response to acclimation to cold. Am. J. Physiol. 239, E301–E309 (1980).
Friedman, R. C., Farh, K. K., Burge, C. B. & Bartel, D. P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 (2009).
Schulz, T. J. et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl Acad. Sci. USA 108, 143–148 (2010).
Payne, V. A. et al. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 425, 215–223 (2009).
Blumberg, J. M. et al. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 281, 11205–11213 (2006).
Rochford, J. J. et al. ETO/MTG8 is an inhibitor of C/EBPβ activity and a regulator of early adipogenesis. Mol. Cell Biol. 24, 9863–9872 (2004).
Timmons, J. A. et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl Acad. Sci. USA 104, 4401–4406 (2007).
Cole, F., Zhang, W., Geyra, A., Kang, J. S. & Krauss, R. S. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell 7, 843–854 (2004).
Kang, J. S., Mulieri, P. J., Miller, C., Sassoon, D. A. & Krauss, R. S. CDO, a robo-related cell surface protein that mediates myogenic differentiation. J. Cell Biol. 143, 403–413 (1998).
Kang, J. S. et al. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38α/β MAPK activity and myogenic differentiation. J. Cell Biol. 182, 497–507 (2008).
Ren, H., Yin, P. & Duan, C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J. Cell Biol. 182, 979–991 (2008).
Teboul, L. et al. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J. Biol. Chem. 270, 28183–28187 (1995).
Seale, P. et al. Transcriptional control of brown fat determination by Prdm16. Cell Metab. 6, 38–54 (2007).
Kajimura, S. et al. Regulation of the brown and white fat gene programs through a Prdm16/CtBP transcriptional complex. Genes Dev. 22, 1397–1409 (2008).
Barbera, M. J. et al. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J. Biol. Chem. 276, 1486–1493 (2001).
Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology 137, 354–366 (1996).
Tong, Y. et al. Suppression of expression of muscle-associated proteins by PPARα in brown adipose tissue. Biochem. Biophys. Res. Commun. 336, 76–83 (2005).
Cannon, B. & Nedergaard, J. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol. Biol. 155, 213–224 (2001).
Tseng, Y. H., Ueki, K., Kriauciunas, K. M. & Kahn, C. R. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J. Biol. Chem. 277, 31601–31611 (2002).
Rodbell, M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239, 375–380 (1964).
Zhang, J., Socolovsky, M., Gross, A. W. & Lodish, H. F. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: Functional analysis by a flow cytometry-based novel culture system. Blood 102, 3938–3946 (2003).
Xie, H., Lim, B. & Lodish, H. F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58, 1050–1057 (2009).
de Hoon, M. J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Dai, M. et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 (2005).
Acknowledgements
This work is supported by NIH grants DK047618, DK 068348, DK076848 and 5P01 HL066105, grant C-382-641-001-091 from the Singapore–MIT Alliance (SMA) and a graduate fellowship from SMA. Thanks for intellectual support, materials and advice from members of the laboratories of Drs P. Seale, D. Bartel and C. Emerson, and from all members of the Lodish laboratory. Thanks to Rochford’s laboratory for their generous gifts of Runx1t1 plasmid.
Author information
Authors and Affiliations
Contributions
L.S., H.X. and H.F.L. conceived the project and designed the experiments. L.S., H.X., M.A.M., R.A., B.Y., S.M.H. and Q.L. carried out the experiments. All authors analysed data. L.S., H.X., M.A.M. and H.F.L. wrote the manuscript. C.R.K. and H.F.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1405 kb)
Rights and permissions
About this article
Cite this article
Sun, L., Xie, H., Mori, M. et al. Mir193b–365 is essential for brown fat differentiation. Nat Cell Biol 13, 958–965 (2011). https://doi.org/10.1038/ncb2286
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2286
This article is cited by
-
Pulling the trigger: Noncoding RNAs in white adipose tissue browning
Reviews in Endocrine and Metabolic Disorders (2024)
-
Key circRNAs from goat: discovery, integrated regulatory network and their putative roles in the differentiation of intramuscular adipocytes
BMC Genomics (2023)
-
An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications
Reviews in Endocrine and Metabolic Disorders (2023)
-
RUNX1T1 function in cell fate
Stem Cell Research & Therapy (2022)
-
miR-375 is cold exposure sensitive and drives thermogenesis in visceral adipose tissue derived stem cells
Scientific Reports (2022)