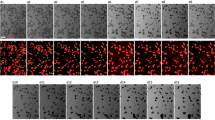
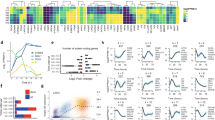
Abstract
Self-renewal of rodent embryonic stem cells is enhanced by partial inhibition of glycogen synthase kinase-3 (Gsk3; refs 1, 2). This effect has variously been attributed to stimulation of Wnt signalling by β-catenin1, stabilization of Myc protein3 and global de-inhibition of anabolic processes4. Here we demonstrate that β-catenin is not necessary for embryonic stem cell identity or expansion, but its absence eliminates the self-renewal response to Gsk3 inhibition. Responsiveness is fully restored by truncated β-catenin lacking the carboxy-terminal transactivation domain5. However, requirement for Gsk3 inhibition is dictated by expression of T-cell factor 3 (Tcf3) and mediated by direct interaction with β-catenin. Tcf3 localizes to many pluripotency genes6 in embryonic stem cells. Our findings confirm that Tcf3 acts as a transcriptional repressor and reveal that β-catenin directly abrogates Tcf3 function. We conclude that Gsk3 inhibition stabilizes the embryonic stem cell state primarily by reducing repressive influence on the core pluripotency network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
27 March 2012
In the version initially published, the received date was incorrect. The correct received date is 20 May 2010.
References
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 (2004).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Cartwright, P. et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132, 885–896 (2005).
Doble, B. W. & Woodgett, J. R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Hsu, S. C., Galceran, J. & Grosschedl, R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β -catenin. Mol. Cell Biol. 18, 4807–4818 (1998).
Cole, M. F., Johnstone, S. E., Newman, J. J., Kagey, M. H. & Young, R. A. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 22, 746–755 (2008).
Clevers, H. Wnt/β -catenin signaling in development and disease. Cell 127, 469–480 (2006).
Behrens, J. et al. Functional interaction of β -catenin with the transcription factor LEF-1. Nature 382, 638–642 (1996).
Molenaar, M. et al. XTcf-3 transcription factor mediates β -catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996).
van de Wetering, M. et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997).
Aberle, H., Bauer, A., Stappert, J., Kispert, A. & Kemler, R. β -catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 (1997).
Rubinfeld, B. et al. Binding of GSK3β to the APC- β -catenin complex and regulation of complex assembly. Science 272, 1023–1026 (1996).
Hanna, J. et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513–524 (2009).
Ogawa, K., Nishinakamura, R., Iwamatsu, Y., Shimosato, D. & Niwa, H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem. Biophys. Res. Commun. 343, 159–166 (2006).
Matsuda, T. et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18, 4261–4269 (1999).
Niwa, H., Burdon, T., Chambers, I. & Smith, A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060 (1998).
Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30–43 (1999).
Pereira, L., Yi, F. & Merrill, B. J. Repression of nanog gene transcription by tcf3 limits embryonic stem cell self-renewal. Mol. Cell Biol. 26, 7479–7491 (2006).
Guo, G., Huang, Y., Humphreys, P., Wang, X. & Smith, A. A PiggyBac-based recessive screening method to identify pluripotency regulators. PLoS One 18, e18189 (2011).
Haegel, H. et al. Lack of β -catenin affects mouse development at gastrulation. Development 121, 3529–3537 (1995).
Huelsken, J. et al. Requirement for β -catenin in anterior–posterior axis formation in mice. J. Cell Biol. 148, 567–578 (2000).
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003).
Bain, J. et al. The selectivity of protein kinase inhibitors; a further update. Biochem. J. 408, 297–315 (2007).
Doble, B. W., Patel, S., Wood, G. A., Kockeritz, L. K. & Woodgett, J. R. Functional redundancy of GSK- 3α and GSK- 3β in Wnt/ β -catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 (2007).
Lyashenko, N. et al. Differential requirement for the dual functions of β -catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. doi:10.1038/ncb2260 (2011).
Brons, I. G. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007).
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
Soncin, F. et al. Abrogation of E-cadherin mediated cell–cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 27, 2069–2080 (2009).
Anton, R., Kestler, H. A. & Kuhl, M. β -Catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 581, 5247–5254 (2007).
Arnold, S. J. et al. Brachyury is a target gene of the Wnt/ β -catenin signaling pathway. Mech. Dev. 91, 249–258 (2000).
Wray, J., Kalkan, T. & Smith, A. G. The ground state of pluripotency. Biochem. Soc. Trans. 38, 1027–1032 (2010).
Li, X. et al. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 273, 34970–34975 (1998).
Toyooka, Y., Shimosato, D., Murakami, K., Takahashi, K. & Niwa, H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135, 909–918 (2008).
Robson, P., Stein, P., Zhou, B., Schultz, R. M. & Baldwin, H. S. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev. Biol. 234, 317–329 (2001).
Yi, F., Pereira, L. & Merrill, B. J. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells 26, 1951–1960 (2008).
Tam, W. L. et al. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells 26, 2019–2031 (2008).
Kelly, K. F. et al. β -catenin enhances Oct-4 activity and reinforcespluripotency through a TCF-independent mechanism. Cell Stem Cell 8, 214–227 (2011).
Moon, R. T. & Kimelman, D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays 20, 536–545 (1998).
ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
Brault, V. et al. Inactivation of the β -catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Acknowledgements
We thank N. Lyashenko and C. Hartmann for discussion and exchange of unpublished data. We also thank A. Martinez-Arias for comments on the manuscript. We are grateful to B. Merrill (University of Illinois at Chicago, USA) for generously providing Tcf3 mutant embryonic stem cells and to B. Doble (Stem Cell and Cancer Research Institute, McMaster University, Canada) and J. Woodgett (Samuel Lunenfeld Research Institute, Mount Sinai Hospital and University of Toronto, Canada) for Gsk3 mutant embryonic stem cells. We thank R. Grosschedl for the β-catenin ΔC construct. Gsk3 inhibitors, compounds A–G, were provided by Pfizer. R. Walker and P. Humphreys supported flow cytometry and imaging, respectively. The study was financially supported by the Biotechnology and Biological Sciences Research Council and the Medical Research Council of the United Kingdom, the Wellcome Trust and the European Commission FP7 project EuroSyStem. S.G-L. was supported by a CONACYT Studentship. A.S. is a Medical Research Council Professor.
Author information
Authors and Affiliations
Contributions
J.W. carried out, analysed and interpreted experiments, T.K. created and validated the Rex1GFPd2 reporter, S.G-L. generated Cre–Ires–fluorescent protein plasmids, D.E. and R.K. generated floxed β-catenin embryonic stem cells, A.C. selected and provided Gsk3 inhibitors, and A.S. supervised the study and wrote the paper together with J.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1790 kb)
Rights and permissions
About this article
Cite this article
Wray, J., Kalkan, T., Gomez-Lopez, S. et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13, 838–845 (2011). https://doi.org/10.1038/ncb2267
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2267
This article is cited by
-
The Wnt/TCF7L1 transcriptional repressor axis drives primitive endoderm formation by antagonizing naive and formative pluripotency
Nature Communications (2023)
-
Ascl1 and Ngn2 convert mouse embryonic stem cells to neurons via functionally distinct paths
Nature Communications (2023)
-
Identification of X-chromosomal genes that drive sex differences in embryonic stem cells through a hierarchical CRISPR screening approach
Genome Biology (2021)
-
STARR-seq identifies active, chromatin-masked, and dormant enhancers in pluripotent mouse embryonic stem cells
Genome Biology (2020)
-
Wnt/Beta-catenin/Esrrb signalling controls the tissue-scale reorganization and maintenance of the pluripotent lineage during murine embryonic diapause
Nature Communications (2020)